Mylepsinum




Mylepsinum Brand names, Mylepsinum Analogs
- Apo-Primidone
- Cyral
- Desoxyphenobarbitone
- Hexadiona
- Hexamidine
- Lepimidin
- Lepsiral
- Liskantin
- Majsolin
- Medi-Pets
- Midone
- Milepsin
- Misodine
- Misolyne
- Mizodin
- Mizolin
- Myidone
- Mylepsin
- Mylepsinum
- Mysedon
- Mysoline
- Neurosyn
- Pms Primidone
- Prilepsin
- Primacione
- Primaclone
- Primacone
- Primakton
- Primidon
- Primidone Methanol Solution
- Primoline
- Prysoline
- Pyrimidone "Medi-Pets"
- Pyrimidone Medi-Pets
- Resimatil
- Sertan
Mylepsinum Brand Names Mixture
- No information avaliable
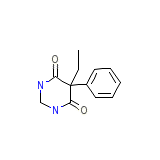
Mylepsinum Chemical_Formula
C12H14N2O2
Mylepsinum RX_link
http://www.rxlist.com/cgi/generic2/primid.htm
Mylepsinum fda sheet
Mylepsinum msds (material safety sheet)
Mylepsinum Synthesis Reference
Boon et al., Brit. pat. 666,027 (1952 to I.C.I.)
Mylepsinum Molecular Weight
218.252 g/mol
Mylepsinum Melting Point
281.5 oC
Mylepsinum H2O Solubility
500 mg/L
Mylepsinum State
Solid
Mylepsinum LogP
1.713
Mylepsinum Dosage Forms
Tablet; Chewable tablets; Oral suspension
Mylepsinum Indication
For the treatment of epilepsy
Mylepsinum Pharmacology
Primidone is a barbiturate with anticonvulsant properties. Primidone, either alone or used concomitantly with other anticonvulsants, is indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy. Primidone raises electro- or chemoshock seizure thresholds or alters seizure patterns in experimental animals. Primidone per se has anticonvulsant activity as do its two metabolites, phenobarbital and phenylethylmalonamide (PEMA). In addition to its anticonvulsant activity, Primidone potentiates that of phenobarbital in experimental animals.
Mylepsinum Absorption
90 to 100%
Mylepsinum side effects and Toxicity
No information avaliable
Mylepsinum Patient Information
Mylepsinum Organisms Affected
Humans and other mammals