Hemabate




Hemabate Brand names, Hemabate Analogs
Hemabate Brand Names Mixture
- No information avaliable
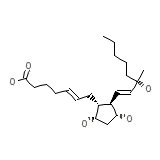
Hemabate Chemical_Formula
C21H36O5
Hemabate RX_link
http://www.rxlist.com/cgi/generic/hemabate.htm
Hemabate fda sheet
Hemabate msds (material safety sheet)
Hemabate Synthesis Reference
No information avaliable
Hemabate Molecular Weight
368.508 g/mol
Hemabate Melting Point
95-105 oC
Hemabate H2O Solubility
Carboprost tromethamine dissolves readily in water at room temperature at a concentration greater than 75 mg/mL.
Hemabate State
Solid
Hemabate LogP
2.578
Hemabate Dosage Forms
Injection (containing 250µg/mL carboprost, 83µg/mL tromethamine).
Hemabate Indication
For aborting pregnancy between the 13th and 20th weeks of gestation as calculated from the first day of the last normal menstrual period and in the following conditions related to second trimester abortion: 1. Failure of expulsion of the fetus during the course of treatment by another method; 2. Premature rupture of membranes in intrauterine methods with loss of drug and insufficient or absent uterine activity; 3. Requirement of a repeat intrauterine instillation of drug for expulsion of the fetus; 4. Inadvertent or spontaneous rupture of membranes in the presence of a previable fetus and absence of adequate activity for expulsion. Also for the treatment of postpartum hemorrhage due to uterine atony which has not responded to conventional methods of management.
Hemabate Pharmacology
Carboprost tromethamine administered intramuscularly stimulates in the gravid uterus myometrial contractions similar to labor contractions at the end of a full term pregnancy. Whether or not these contractions result from a direct effect of carboprost on the myome-trium has not been determined. Nonetheless, they evacuate the products of conception from the uterus in most cases. Postpartum, the resultant myometrial contractions provide hemostasis at the site of placentation. Carboprost tromethamine also stimulates the smooth muscle of the human gastrointestinal tract. This activity may produce the vomiting or diarrhea or both that is common when carbo-prost tromethamine is used to terminate pregnancy and for use postpartum. In laboratory animals and also in humans carboprost tromethamine can elevate body temperature. With the clinical doses of carboprost trometh-amine used for the termination of pregnancy, and for use postpartum, some patients do experience transient temperature increases. In laboratory animals and in humans large doses of carboprost tromethamine can raise blood pressure, probably by contracting the vascular smooth muscle. With the doses of carboprost tromethamine used for terminating pregnancy, this effect has not been clinically significant. In laboratory animals and also in humans carboprost tromethamine can elevate body temperature. With the clinical doses of carboprost tromethamine used for the termination of pregnancy, some patients do experience temperature increases. In some patients, carboprost tromethamine may cause transient bronchoconstriction.
Hemabate Absorption
No information avaliable
Hemabate side effects and Toxicity
Symptoms of overdose include irritation, nausea, vomiting, diarrhea, coughing, dyspnea, asthma, hypertension, flushing, and pyrexia.
Hemabate Patient Information
Hemabate Organisms Affected
Humans and other mammals