Fatroximin




Fatroximin Brand names, Fatroximin Analogs
Fatroximin Brand Names Mixture
- Sans-Acne Solution (Alcohol Anhydrous + Erythromycin)
- Staticin Lot (Alcohol Anhydrous + Erythromycin + Laureth 4)
- T-Stat Lot (Alcohol Anhydrous + Erythromycin)
- T-Stat Pad-Lot (Alcohol Anhydrous + Erythromycin)
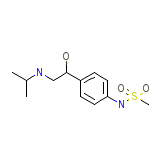
Fatroximin Chemical_Formula
C12H20N2O3S
Fatroximin RX_link
http://www.rxlist.com/cgi/generic/betapaceaf.htm
Fatroximin fda sheet
Fatroximin msds (material safety sheet)
Fatroximin Synthesis Reference
No information avaliable
Fatroximin Molecular Weight
272.365 g/mol
Fatroximin Melting Point
206.5-207 oC
Fatroximin H2O Solubility
Soluble (5510 mg/L)
Fatroximin State
Solid
Fatroximin LogP
2.382
Fatroximin Dosage Forms
Tablets (white, capsule-shaped, for oral administration)
Fatroximin Indication
For the maintenance of normal sinus rhythm [delay in time to recurrence of atrial fibrillation/atrial flutter (AFIB/AFL)] in patients with symptomatic AFIB/AFL who are currently in sinus rhythm. Also for the treatment of documented life-threatening ventricular arrhythmias.
Fatroximin Pharmacology
Sotalol is an antiarrhythmic drug. It falls into the class of beta blockers (and class II antiarrhythmic agents) because of its primary action on the β-adrenergic receptors in the heart. In addition to its actions on the beta receptors in the heart, sotalol inhibits the inward potassium ion channels of the heart. In so doing, sotalol prolongs repolarization, therefore lengthening the QT interval and decreasing automaticity. It also slows atrioventricular (AV) nodal conduction. Because of these actions on the cardiac action potential, it is also considered a class III antiarrhythmic agent. The beta-blocking effect of sotalol is non-cardioselective, half maximal at about 80mg/day and maximal at doses between 320 and 640 mg/day. Sotalol does not have partial agonist or membrane stabilizing activity. Although significant beta-blockade occurs at oral doses as low as 25 mg, significant Class Ieffects are seen only at daily doses of 160 mg and above.
Fatroximin Absorption
In healthy subjects, the oral bioavailability of sotalol is 90-100%. Absorption is reduced by approximately 20% compared to fasting when administered with a standard meal.
Fatroximin side effects and Toxicity
The most common signs to be expected are bradycardia, congestive heart failure, hypotension, bronchospasm and hypoglycemia. In cases of massive intentional overdosage (2-16 grams) of sotalol the following clinical findings were seen: hypotension, bradycardia, cardiac asystole, prolongation of QT interval, Torsade de Pointes, ventricular tachy-cardia, and premature ventricular complexes.
Fatroximin Patient Information
Fatroximin Organisms Affected
Humans and other mammals