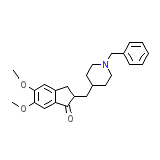
Donepezil




Donepezil Brand names, Donepezil Analogs
Donepezil Brand Names Mixture
- No information avaliable
Donepezil Chemical_Formula
C24H29NO3
Donepezil RX_link
http://www.rxlist.com/cgi/generic/donepezil.htm
Donepezil fda sheet
Donepezil msds (material safety sheet)
Donepezil Synthesis Reference
No information avaliable
Donepezil Molecular Weight
379.492 g/mol
Donepezil Melting Point
206.72 oC
Donepezil H2O Solubility
2.931 mg/L
Donepezil State
Solid
Donepezil LogP
4.09
Donepezil Dosage Forms
Tablet; Tablet (orally disintegrating)
Donepezil Indication
For management of symptoms associated with Alzheimer's Disease
Donepezil Pharmacology
Donepezil is a centrally acting reversible acetyl cholinesterase inhibitor. Its main therapeutic use is in the treatment of Alzheimer's disease where it is used to increase cortical acetylcholine. It is well absorbed in the gut with an oral bioavailability of 100% and easily crosses the blood-brain barrier. Because it has a half life of about 70 hours, it can be taken once a day. Initial dose is 5 mg per day, which can be increased to 10 mg per day after an adjustment period of at least 4 weeks. Donepezil is a parasympathomimetic, specifically, a reversible cholinesterase inhibitor. Donepezil is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase. If this proposed mechanism of action is correct, donepezil's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that donepezil alters the course of the underlying dementing process.
Donepezil Absorption
Donepezil is well absorbed with a relative oral bioavailability of 100% and reaches peak plasma concentrations in 3 to 4 hours.
Donepezil side effects and Toxicity
Symptoms of overdose include severe nausea, vomiting, salivation, sweating, bradycardia, hypotension, respiratory depression, collapse and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved.
Donepezil Patient Information
Patient Info
ARICEPT� (donepezil HCl) is indicated for the treatment of symptoms of mild to moderate Alzheimer�s disease.
ARICEPT� (donepezil HCl) is well tolerated but may not be for everyone. Some people may experience nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue or loss of appetite. In studies, these side effects were usually mild and temporary. Some people taking ARICEPT� (donepezil HCl) may experience fainting. People at risk for ulcers should tell their doctors because their condition may get worse.
ARICEPT� (donepezil HCl) is indicated for the treatment of symptoms of mild to moderate Alzheimer�s disease.
ARICEPT� (donepezil HCl) is well tolerated but may not be for everyone. Some people may experience nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue or loss of appetite. In studies, these side effects were usually mild and temporary. Some people taking ARICEPT� (donepezil HCl) may experience fainting. People at risk for ulcers should tell their doctors because their condition may get worse.
Donepezil Organisms Affected
Humans and other mammals