CAP




CAP Brand names, CAP Analogs
- Biopolymers
- Copegus
- RBV
- RIBAV
- RTC
- RTCA
- RTP
- Rebetol
- Rebetron
- Rebretron
- Ribamide
- Ribamidil
- Ribamidyl
- Ribasphere
- Ribavirin Capsules
- Ribavirin Triphosphate
- Ribavirin [Usan:Inn]
- Ribavirin-TP
- Ribavirina [Inn-Spanish]
- Ribavirine
- Ribavirine [Inn-French]
- Ribavirinum [Inn-Latin]
- Tribavirin
- Varazid
- Vilona
- Viramid
- Virazid
- Virazole
- Virazole 5'-triphosphate
CAP Brand Names Mixture
- Rebetron (Ribavirin + Interferon alpha)
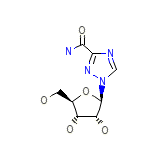
CAP Chemical_Formula
CAP RX_link
CAP fda sheet
CAP msds (material safety sheet)
CAP Synthesis Reference
CAP Molecular Weight
CAP Melting Point
CAP H2O Solubility
CAP State
CAP LogP
CAP Dosage Forms
CAP Indication
CAP Pharmacology
CAP Absorption
CAP side effects and Toxicity
CAP Patient Information
Patients must be informed that ribavirin may cause birth defects and/or death of the exposed fetus. COPEGUS therapy must not be used by women who are pregnant or by men whose female partners are pregnant. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients taking COPEGUS therapy and for 6 months posttherapy. COPEGUS therapy should not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Patients must perform a pregnancy test monthly during therapy and for 6 months posttherapy.
Female patients of childbearing potential and male patients with female partners of childbearing potential must be advised of the teratogenic/embryocidal risks and must be instructed to practice effective contraception during COPEGUS therapy and for 6 months posttherapy. Patients should be advised to notify the healthcare provider immediately in the event of a pregnancy.
The most common adverse event associated with ribavirin is anemia, which may be severe. Patients should be advised that laboratory evaluations are required prior to starting COPEGUS therapy and periodically thereafter. It is advised that patients be well hydrated, especially during the initial stages of treatment.
Patients who develop dizziness, confusion, somnolence, and fatigue should be cautioned to avoid driving or operating machinery.
Patients should be informed regarding the potential benefits and risks attendant to the use of COPEGUS. Instructions on appropriate use should be given, including review of the contents of the enclosed MEDICATION GUIDE, which is not a disclosure of all or possible adverse effects.
Patients should be advised to take COPEGUS with food.
MEDICATION GUIDE
COPEGUS®
(ribavirin, USP)
TABLETS
Read this Medication Guide carefully before you start taking COPEGUS and read the Medication Guide each time you get more COPEGUS. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about COPEGUS?
1. COPEGUS, a form of ribavirin, may cause birth defects or death of an unborn child. Therefore, if you are pregnant or your partner is pregnant or plans to become pregnant, do not take COPEGUS. Female patients and female partners of male patients being treated with COPEGUS must not become pregnant during treatment and for 6 months after treatment has stopped.
During this time you must have pregnancy tests that show you are not pregnant. You must also use 2 effective forms of birth control during therapy and for 6 months after stopping therapy. Male patients should use a condom with spermicide as one of the two forms.
If pregnancy occurs, report the pregnancy to your healthcare provider right away.
If you or a female sexual partner becomes pregnant, you should tell your healthcare provider. There is a Ribavirin Pregnancy Registry that collects information about pregnancy outcomes of female patients and female partners of male patients exposed to ribavirin. You or your healthcare provider are encouraged to contact the Registry at 1-800-593-2214.
2. COPEGUS can cause a dangerous drop in your red blood cell count. COPEGUS can cause anemia, which is a decrease in the number of red blood cells. This can be dangerous, especially if you have heart or breathing problems. This may cause a worsening of heart (cardiovascular) or circulatory problems. Some patients may get chest pain and rarely, a heart attack. Patients with a history of heart disease have the highest chance of this. Tell your healthcare provider, before taking COPEGUS if you have or have ever had any heart or breathing problems. Your healthcare provider should check your red blood cell count before you start treatment with COPEGUS and often during the first 4 weeks of treatment. Your red blood cell count may be done more often if you have any heart or breathing problems.
3. Do not take COPEGUS alone to treat hepatitis C virus infection. COPEGUS does not treat hepatitis C virus infections by itself. COPEGUS should be used in combination with PEGASYS® (peginterferon alfa-2a) to treat continuing (chronic) hepatitis C virus infections. You should read the Medication Guide for PEGASYS because it has additional important information about treatment that is not covered in this Medication Guide. Your healthcare provider or pharmacist should give you a copy of the PEGASYS Medication Guide.
What is COPEGUS?
COPEGUS is the antiviral medicine ribavirin. It is used in combination with a medicine called PEGASYS (peginterferon alfa-2a) to treat some adults with chronic hepatitis C whose liver still works normally, and who have not been treated before with a medicine called an interferon alpha. It is not known how COPEGUS and PEGASYS work together to fight hepatitis C virus infections.
It is not known if treatment with COPEGUS and PEGASYS combination therapy can cure hepatitis C or if it can prevent liver damage (cirrhosis), liver failure or liver cancer that is caused by hepatitis C virus infections. It is not known if treatment with COPEGUS and PEGASYS combination therapy will prevent an infected person from spreading the hepatitis C virus to another person.
Treatment with COPEGUS has not been studied in children under 18 years of age.
Who should not take COPEGUS?
Do not use COPEGUS if:
· You are a female and you are pregnant or plan to become pregnant during treatment or during the 6 months after your treatment has ended.
· You are a male patient with a female sexual partner who is pregnant or plans to become pregnant at any time while you are being treated with COPEGUS or during the 6 months after your treatment has ended.
· You are breast feeding. We do not know if COPEGUS can pass through your milk and if it can harm your baby. You will need to choose either to breast-feed or take COPEGUS, but not both.
· You have a liver disease called autoimmune hepatitis (hepatitis caused by your immune system attacking your liver).
· You have unstable or severe liver disease.
· You are allergic to any of the ingredients in COPEGUS. The active ingredient in COPEGUS is ribavirin. See the end of this Medication Guide for a list of all the ingredients in COPEGUS.
Tell your healthcare provider before starting treatment with COPEGUS in combination with PEGASYS if you have any of the following medical conditions:
· mental health problems, such as depression or anxiety: COPEGUS and PEGASYS combination therapy may make them worse. Tell your healthcare provider if you are being treated or had treatment in the past for any mental problems, including depression, thoughts of ending your life (suicidal thoughts) or a feeling of loss of contact with reality, such as hearing voices or seeing things that are not there (psychosis). Tell your healthcare provider if you take any medicines for these problems.
· high blood pressure, heart problems or have had a heart attack. COPEGUS may worsen heart problems such as high blood pressure, increased heart rate, and chest pain. Tell your healthcare provider if you have or had a heart problem. Patients who have had certain heart problems should not take COPEGUS.
· blood disorders, including anemia (low red blood cell count), thalassemia (Mediterranean anemia) and sickle-cell anemia. COPEGUS can reduce the number of red blood cells you have. This may make you feel dizzy or weak and could worsen any heart problems you might have.
· kidney problems. If your kidneys do not work properly, you may have worse side effects from COPEGUS treatment and require a lower dose.
· liver problems (other than hepatitis C virus infection).
· organ transplant, and you are taking medicine that keeps your body from rejecting your transplant (suppresses your immune system).
· thyroid disease. COPEGUS and PEGASYS combination therapy may make your thyroid disease worse or harder to treat. COPEGUS and PEGASYS treatment may be stopped if you develop thyroid problems that cannot be controlled by medicine.
· have or had drug or alcohol addiction or abuse.
· cancer.
· infection with hepatitis B virus.
· diabetes. COPEGUS and PEGASYS combination therapy may make your diabetes worse or harder to treat.
· past interferon treatment for hepatitis C virus infection that did not work for you.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins or herbal supplements. Some medicines can cause serious side effects if taken while you also take COPEGUS. Some medicines may affect how COPEGUS works or COPEGUS may affect how your other medicines work. Be especially sure to tell your healthcare provider if you take any medicines to treat HIV.
For more information see the PEGASYS Medication Guide.
How should I take COPEGUS?
· Your healthcare provider will determine the right dose of COPEGUS based on your weight.
· Take COPEGUS 1 time in the morning and 1 time at night (2 times a day). Take COPEGUS the same 2 times each day.
· Take COPEGUS with food.
· It is very important to follow your dosing schedule and your healthcare provider’s instructions on how to take your medicines.
· Take COPEGUS for as long as it is prescribed, and do not take more than your healthcare provider prescribes.
· If you miss a dose of COPEGUS and remember the same day, take the missed dose as soon as you remember. If the whole day has passed, ask your healthcare provider what to do. Do not take 2 doses at the same time.
· Your healthcare provider may adjust your dose of COPEGUS based on blood tests that show your response to treatment and side effects you may have.
· Females taking COPEGUS or female sexual partners of male patients taking COPEGUS must have a pregnancy test:
· before treatment begins
· every month during treatment
· for 6 months after treatment ends to make sure there is no pregnancy
It is also important not to use other ribavirin medicines without talking to your healthcare provider. Please see the PEGASYS Medication Guide for the proper use of PEGASYS injection.
What should I avoid while taking COPEGUS?
Avoid the following during COPEGUS treatment:
· Do not get pregnant. If you or your sexual partner get pregnant during treatment with COPEGUS or in the 6 months after treatment ends, tell your healthcare provider right away.
Talk with your healthcare provider about birth control methods and how to avoid pregnancy. You must use extreme care to avoid pregnancy during and for 6 months after treatment in female and male patients.
· Do not take COPEGUS alone to treat your hepatitis C virus infection. COPEGUS should be used in combination with PEGASYS (peginterferon alfa-2a) to treat chronic hepatitis C virus infections.
· Do not breast feed. COPEGUS may pass through your milk and may harm your baby.
· Do not drink alcohol, including beer, wine, and liquor. This may make your liver disease worse.
· Do not drive or operate machinery if COPEGUS makes you feel tired, dizzy or confused.
· Do not take other medicines unless your healthcare provider knows about them. Take only medicines prescribed or approved by your healthcare provider. These include prescription and non-prescription medicines, vitamins or herbal supplements. Talk to your healthcare provider before starting any new medicine.
What are the possible side effects of COPEGUS?
The most serious possible side effects of COPEGUS are:
· Harm to unborn children. COPEGUS may cause birth defects or death of an unborn child. (For more details, see “What is the most important information I should know about COPEGUS?” .)
· Anemia. Anemia is a reduction in the number of red blood cells you have. Anemia can be dangerous, especially if you have heart or breathing problems. Tell your healthcare provider right away if you feel tired, have chest pain or shortness of breath. These may be signs of low red blood cell counts.
· Liver Problems. Some patients may develop worsening of liver function. Some of the symptoms may include stomach bloating, confusion, brown urine, and yellow eyes. Tell your healthcare provider immediately if any of these symptoms occur.
Call your healthcare provider right away if you have any of the following symptoms. They may be signs of a serious side effect of COPEGUS and PEGASYS treatment.
· trouble breathing
· hives or swelling
· chest pain
· severe stomach pain or low back pain
· bloody diarrhea or bloody stools (bowel movements). These may look like black tar.
· bruising or unusual bleeding
· change in your vision
· high fever (temperature greater than 100.5°F)
· you have psoriasis (a skin disease) and it gets worse
· you become very depressed or think about suicide (ending your life)
The most common side effects of COPEGUS are likely to be the same as for other ribavirin products. These are:
· feeling tired
· nausea and appetite loss
· rash and itching
· cough
These are not all the possible side effects of COPEGUS treatment. For more information, ask your doctor or pharmacist and see the PEGASYS Medication Guide.
What should I know about hepatitis C infection?
Hepatitis C infection is a disease caused by a virus that infects the liver. Hepatitis C is more serious for some people than others. Most people who get hepatitis C carry the virus in their blood for the rest of their lives. Most of these people will have some liver damage, but many do not feel sick from the disease. In some people, the liver becomes badly damaged and scarred. This is called cirrhosis. Cirrhosis can cause the liver to stop working. Some people may get liver cancer or liver failure from the hepatitis C virus.
Hepatitis C virus is spread from one person to another by contact with an infected person. s blood. You should talk to your healthcare provider about ways to prevent you from infecting others.
How should I store COPEGUS?
Store COPEGUS tablets at room temperature (77°F).
Please refer to the PEGASYS Medication Guide for storage information about PEGASYS injection.
General information about the safe and effective use of COPEGUS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use COPEGUS for a condition for which it was not prescribed. Do not give COPEGUS to other people, even if they have the same symptoms that you have.
This Medication Guide summarizes the most important information about COPEGUS. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about COPEGUS that is written for healthcare professionals.
What are the ingredients in COPEGUS?
Active Ingredient: ribavirin
Inactive Ingredients: pregelatinized starch, sodium starch glycolate, cornstarch, microcrystalline cellulose, and magnesium stearate. The tablet is coated with aquacoat ECD-30, triacetin, and colored with a coating system composed of hydroxypropyl methyl cellulose, talc, titanium dioxide, synthetic yellow iron oxide, and synthetic red iron oxide.
This Medication Guide has been approved by the US Food and Drug Administration.
Revised: February 2005
Distributed by: Roche Pharmaceuticals Roche Laboratories Inc. 340 Kingsland Street Nutley, New Jersey 07110-1199 27898884 Copyright© 2002-2005 by Roche Laboratories Inc. All rights reserved.