Acediur




Acediur Brand names, Acediur Analogs
Acediur Brand Names Mixture
- No information avaliable
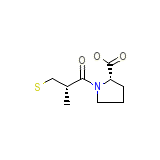
Acediur Chemical_Formula
Acediur RX_link
Acediur fda sheet
Acediur msds (material safety sheet)
Acediur Synthesis Reference
Acediur Molecular Weight
Acediur Melting Point
Acediur H2O Solubility
Acediur State
Acediur LogP
Acediur Dosage Forms
Acediur Indication
Acediur Pharmacology
Acediur Absorption
Acediur side effects and Toxicity
Acediur Patient Information
Patients should be advised to immediately report to their physician any signs or symptoms suggesting angioedema (e.g., swelling of face, eyes, lips, tongue, larynx and extremities; difficulty in swallowing or breathing; hoarseness) and to discontinue therapy.
Patients should be told to report promptly any indication of infection (e.g., sore throat, fever),which may be a sign of neutropenia, or of progressive edema which might be related to proteinuria and nephrotic syndrome.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with the physician.
Patients should be advised not to use potassium-sparing diuret-ics, potassium supplements or potassium-containing salt substitutes without consulting their physician.
Patients should be warned against interruption or discontinuation of medication unless instructed by the physician.
Heart failure patients on captopril therapy should be cautioned against rapid increases in physical activity.
Patients should be informed that captopril should be taken one hour before meals.