Vistide




Categoria
Vistide Marchi, Vistide Analoghi
Vistide Marchi miscela
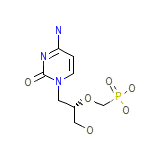
Vistide Formula chimica
C8H14N3O6P
Vistide RX link
http://www.rxlist.com/cgi/generic2/cidofovir.htm
Vistide FDA foglio
Vistide DMS (foglio di materiale di sicurezza)
Vistide Sintesi di riferimento
Nessuna informazione disponibile
Vistide Peso molecolare
279.187 g/mol
Vistide Temperatura di fusione
480 oC
Vistide H2O Solubilita
Vistide Stato
Solid
Vistide LogP
-3.3
Vistide Forme di dosaggio
Parenterale
Vistide Indicazione
Per il trattamento della retinite da CMV nei pazienti con sindrome da immunodeficienza acquisita (AIDS)
Vistide Farmacologia
Cidofovir è un nuovo farmaco anti-virale. E 'classificato come un analogo nucleotidico ed è attiva contro l'herpes citomegalovirus (CMV) retinite. Maggior parte degli adulti con infezione da CMV. Cidofovir sopprime citomegalovirus (CMV) replica inibizione selettiva della sintesi del DNA virale. I dati biochimici confermano l'inibizione selettiva della DNA polimerasi CMV mediante cidofovir difosfato, la intracellulare attivo metabolita di cidofovir. Cidofovir difosfato inibisce la polimerasi herpesvirus a concentrazioni che sono 8 - a 600 volte inferiori a quelle necessarie per inibire le cellule umane DNA polimerasi alfa, beta e gamma (1,2,3). L'incorporazione del cidofovir nei risultati del DNA virale in crescita a catena in una riduzione del tasso di sintesi del DNA virale.
Vistide Assorbimento
100%
Vistide Tossicita
Danni renali, diminuzione del numero di globuli bianchi, diminuzione delle piastrine
Vistide Informazioni paziente
CIDOFOVIR
Other names: Vistide®
WHY is this medication prescribed ?
Cidofovir is an antiviral medication used for the treatment of Cytomegalovirus (CMV) infection. The drug is also used to prevent a relapse of CMV after the initial treatment of the infection.
HOW should this drug be taken ?
Cidofovir is given intravenously every week for two weeks and then on alternating weeks for an indefinite period of time. The protocol for cidofovir administration is as follows: · 2000 mg oral dose of probenecid (4 x 500 mg tablets) three hours prior to cidofovir
1 liter infusion of saline one hour prior to cidofovir
Cidofovir infusion over one hour.
While cidofovir is infusing , another liter of saline may be given over 1 to 3 hours.
1000 mg (2 x 500 mg tablets) of probenecid 2 hours after the cidofovir infusion is completed
1000 mg (2 x 500 mg tablets) of probenicid 8 hours after the cidofovir infusion is completed.
What ADVERSE EFFECTS can this drug cause? What should you do about them?
The most serious and commonly reported negative effect of intravenous cidofovir is kidney toxicity. Your kidney status will be checked the day before you receive an infusion of cidofovir. If measurements of serum creatinine and protein in your urine are not within acceptable limits, your physician will either reduce your dose or discontinue therapy. Hydration with normal saline and administration of oral probenecid help to reduce the risk of kidney toxicity.
Cidofovir can also cause a fall in the number of white blood cells. This can increase your risk of developing infections as white blood cells are needed to fight infections. Cidofovir can also decrease platelets. This can increase your risk of bleeding or bruising as platelets are need to help your blood clot. Your red blood cells may also decrease which could make you feel tired or short of breath. Blood tests will be done regularly to check for any changes in these values. If there are any serious problems, cidofovir will be stopped. Nausea, fever, hair loss and muscle pain can also occur as a result of cidofovir therapy. If these effects persist and are bothersome, please call the clinic or discuss this at your next visit.
Some side effects experienced by patients treated with cidofovir may be related to probenecid. These side effects are rash, nausea, vomiting and fever. Eating food before taking probenecid helps reduce the risk of nausea and vomiting. Patients who experience rash or itching are usually given an antihistamine to reduce symptoms. Inform you doctor if you experience intolerable side effects.
IF YOU ARE EXPERIENCING ANY ADVERSE EFFECTS PLEASE DISCUSS THEM WITH YOUR HEALTH CARE TEAM.
IT IS IMPORTANT THAT YOU KEEP YOUR REGULAR DOCTOR'S APPOINTMENTS, SO THAT YOUR PROGRESS CAN BE ASSESSED.
What other PRECAUTIONS should you follow while using this drug?
If while handling the drug, it accidentally spills, avoid contact with your skin, eyes, or mucous membranes (eg. nose). Wear rubber gloves while cleaning up any spills. If the drug comes in contact with your skin or mucous membranes, wash the area thoroughly with soap and water, and rinse thoroughly with water.
Once started on cidofovir therapy, it is to be continued indefinitely even if you begin to feel your vision is improving. Maintain you regular visits and inform your doctor of any new medical problems that develop while you are receiving cidofovir.
Birth defects have occurred in animals exposed to cidofovir, therefore cidofovir should be used during pregnancy only if the potential justifies the risk to the fetus. It is not known if cidofovir is excreted into breast milk. Because of abnormalities which have occurred in animals treated with cidofovir, it is possible that nursing infants may also be affected, therefore cidofovir should not be given to breast feeding mothers. If these issues are a concern to you, please discuss this with your doctor or pharmacist.
Other medications may affect the way cidofovir works and so increase the chance of side effects. Also probenecid may affect the way other agents work. For example, if you are taking zidovudine (AZT), you should decrease you daily dose by 50% on the day you receive probenecid/cidofovir. Inform your doctor and pharmacist about all of the medications you are taking, and do not start taking other medications without discussing this first.
Other names: Vistide®
WHY is this medication prescribed ?
Cidofovir is an antiviral medication used for the treatment of Cytomegalovirus (CMV) infection. The drug is also used to prevent a relapse of CMV after the initial treatment of the infection.
HOW should this drug be taken ?
Cidofovir is given intravenously every week for two weeks and then on alternating weeks for an indefinite period of time. The protocol for cidofovir administration is as follows: · 2000 mg oral dose of probenecid (4 x 500 mg tablets) three hours prior to cidofovir
1 liter infusion of saline one hour prior to cidofovir
Cidofovir infusion over one hour.
While cidofovir is infusing , another liter of saline may be given over 1 to 3 hours.
1000 mg (2 x 500 mg tablets) of probenecid 2 hours after the cidofovir infusion is completed
1000 mg (2 x 500 mg tablets) of probenicid 8 hours after the cidofovir infusion is completed.
What ADVERSE EFFECTS can this drug cause? What should you do about them?
The most serious and commonly reported negative effect of intravenous cidofovir is kidney toxicity. Your kidney status will be checked the day before you receive an infusion of cidofovir. If measurements of serum creatinine and protein in your urine are not within acceptable limits, your physician will either reduce your dose or discontinue therapy. Hydration with normal saline and administration of oral probenecid help to reduce the risk of kidney toxicity.
Cidofovir can also cause a fall in the number of white blood cells. This can increase your risk of developing infections as white blood cells are needed to fight infections. Cidofovir can also decrease platelets. This can increase your risk of bleeding or bruising as platelets are need to help your blood clot. Your red blood cells may also decrease which could make you feel tired or short of breath. Blood tests will be done regularly to check for any changes in these values. If there are any serious problems, cidofovir will be stopped. Nausea, fever, hair loss and muscle pain can also occur as a result of cidofovir therapy. If these effects persist and are bothersome, please call the clinic or discuss this at your next visit.
Some side effects experienced by patients treated with cidofovir may be related to probenecid. These side effects are rash, nausea, vomiting and fever. Eating food before taking probenecid helps reduce the risk of nausea and vomiting. Patients who experience rash or itching are usually given an antihistamine to reduce symptoms. Inform you doctor if you experience intolerable side effects.
IF YOU ARE EXPERIENCING ANY ADVERSE EFFECTS PLEASE DISCUSS THEM WITH YOUR HEALTH CARE TEAM.
IT IS IMPORTANT THAT YOU KEEP YOUR REGULAR DOCTOR'S APPOINTMENTS, SO THAT YOUR PROGRESS CAN BE ASSESSED.
What other PRECAUTIONS should you follow while using this drug?
If while handling the drug, it accidentally spills, avoid contact with your skin, eyes, or mucous membranes (eg. nose). Wear rubber gloves while cleaning up any spills. If the drug comes in contact with your skin or mucous membranes, wash the area thoroughly with soap and water, and rinse thoroughly with water.
Once started on cidofovir therapy, it is to be continued indefinitely even if you begin to feel your vision is improving. Maintain you regular visits and inform your doctor of any new medical problems that develop while you are receiving cidofovir.
Birth defects have occurred in animals exposed to cidofovir, therefore cidofovir should be used during pregnancy only if the potential justifies the risk to the fetus. It is not known if cidofovir is excreted into breast milk. Because of abnormalities which have occurred in animals treated with cidofovir, it is possible that nursing infants may also be affected, therefore cidofovir should not be given to breast feeding mothers. If these issues are a concern to you, please discuss this with your doctor or pharmacist.
Other medications may affect the way cidofovir works and so increase the chance of side effects. Also probenecid may affect the way other agents work. For example, if you are taking zidovudine (AZT), you should decrease you daily dose by 50% on the day you receive probenecid/cidofovir. Inform your doctor and pharmacist about all of the medications you are taking, and do not start taking other medications without discussing this first.