Naratriptan




Categoria
Naratriptan Marchi, Naratriptan Analoghi
Naratriptan Marchi miscela
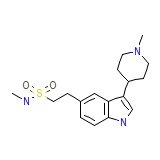
Naratriptan Formula chimica
Naratriptan RX link
Naratriptan FDA foglio
Naratriptan DMS (foglio di materiale di sicurezza)
Naratriptan Sintesi di riferimento
Naratriptan Peso molecolare
Naratriptan Temperatura di fusione
Naratriptan H2O Solubilita
Naratriptan Stato
Naratriptan LogP
Naratriptan Forme di dosaggio
Naratriptan Indicazione
Naratriptan Farmacologia
Naratriptan Assorbimento
Naratriptan Tossicita
Naratriptan Informazioni paziente
Information for the Patient AMERGEô (naratriptan hydrochloride) Tablets
Please read this leaflet carefully before you take AMERGE Tablets. This leaflet provides a summary of the information available about your medicine. Please do not throw away this leaflet until you have finished your medicine. You may need to read this leaflet again. This leaflet does not contain all the information on AMERGE Tablets. For further information or advice, ask your doctor or pharmacist.
Information About Your Medicine
The name of your medicine is AMERGE (naratriptan hydrochloride) Tablets. It can be obtained only by prescription from your doctor. The decision to use AMERGE Tablets is one that you and your doctor should make jointly, taking into account your individual preferences and medical circumstances. If you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male over 40), you should tell your doctor, who should evaluate you for heart disease in order to determine if AMERGE is appropriate for you. The majority of those who have taken AMERGE Tablets have not experienced any significant side effects.
Rarely, deaths and or serious heart problems have been reported with this class of medicines; in all but a few instances, however, these deaths and/ or serious heart problems occurred in people with heart disease and it was not clear whether these medications were a contributing factor.
1. The Purpose of Your Medicine:
AMERGE Tablets are intended to relieve your migraine, but not to prevent or reduce the number of attacks you experience. Use AMERGE Tablets only to treat an actual migraine attack.
2. Important Questions to Consider Before Taking AMERGE Tablets:
If the answer to any of the following questions is YES or if you do not know the answer, then please discuss it with your doctor before you use AMERGE Tablets.
° Are you pregnant? Do you think you might be pregnant? Are you trying to become pregnant? Are you not using adequate contraception? Are you breast-feeding?
° Do you have any chest pain, heart disease, shortness of breath, or irregular heartbeats? Have you had a heart attack?
° Do you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male over 40)?
° Have you had a stroke, transient ischemic attacks or "TIAs", or Raynaud syndrome?
° Do you have high blood pressure?
° Have you ever had to stop taking this or any other medication because of an allergy or other problems?
° Are you taking any other migraine medications, including other 5-HT1 agonists such as IMITREX® (sumatriptan), or medications containing ergotamine, dihydroergotamine, or methysergide?
° Are you taking any medication for depression such as selective serotonin reuptake inhibitors [SSRIs])?
° Have you h.d. or do you have, any disease of the kidney or liver?
° Is this headache different from your usual migraine attacks? Remember, if you answered YES to any of the above questions, then discuss it with your doctor.
3. The Use of AMERGE Tablets During Pregnancy:
Do not use AMERGE Tablets if you are pregnant, think you might be pregnant, are trying to become pregnant, or are not using adequate contraception, unless you have discussed this with your doctor.
4. How to Use AMERGE Tablets:
For adults, the usual dose is a single tablet taken whole with fluids. It may be given at any time after the headache starts. For an individual attack, if you have no response to the first tablet, do not take a second tablet without first talking to your doctor. If you need more relief due to a partial response or return of your headache after the first tablet, a second tablet may be taken but not sooner than 4 hours following the first tablet. Do not take more than a total of two AMERGE Tablets in any 24-hour period. If you have kidney or liver disease, take as directed by your doctor.
5. Side Effects to Watch for:
Some patients experience pain or tightness in the chest or throat when using AMERGE Tablets. If this happens to you, then discuss it with your doctor before using any more AMERGE Tablets. If the chest pain, tightness, or pressure is severe or does not go away, call your doctor immediately.
° If you have sudden and or severe abdominal pain following AMERGE Tablets, call your doctor immediately.
° Shortness of breath; wheeziness; heart throbbing, swelling of eyelids, face, or lips; or a skin rash, skin lumps, or hives happens rarely. If it happens to you, then tell your doctor immediately. Do not take any more AMERGE Tablets unless your doctor tells you to do so.
° Some people may have feelings of tingling, heat, flushing (redness of face lasting a short time), heaviness or pressure after treatment with AMERGE Tablets. A few people may feel drowsy, dizzy, tired, or sick. Tell your doctor of these symptoms at your next visit.
° If you feel unwell in any other way or have any symptoms that you do not understand, you should contact your doctor immediately.
6. What to Do if an Overdose is Taken:
If you have taken more medication than you have been told, contact either your doctor, hospital emergency department, or nearest poison control center immediately.
7. Storing Your Medicine:
Keep your medicine in a safe place where children cannot reach it. It may be harmful to children. Store your medication away from heat and light. Do not store at temperatures above 77° F (25° C). If your medication has expired (the expiration date is printed on the treatment pack), throw it away as instructed. If your doctor decides to stop your treatment, do not keep any leftover medicine unless your doctor tells you to. Throw away your medicine as instructed.