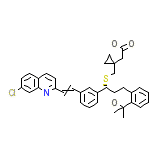
Montelukast




Categoria
Montelukast Marchi, Montelukast Analoghi
Montelukast Marchi miscela
Montelukast Formula chimica
C35H36ClNO3S
Montelukast RX link
http://www.rxlist.com/cgi/generic3/monteluk.htm
Montelukast FDA foglio
Montelukast DMS (foglio di materiale di sicurezza)
Montelukast Sintesi di riferimento
Belley ML et al. Eur. Pat. Appl. 480,717 (1992)
Montelukast Peso molecolare
586.184 g/mol
Montelukast Temperatura di fusione
No information avaliable
Montelukast H2O Solubilita
Nessuna informazione disponibile
Montelukast Stato
Solid
Montelukast LogP
8.488
Montelukast Forme di dosaggio
Tablet (orale)
Montelukast Indicazione
Per il trattamento di asma
Montelukast Farmacologia
Montelukast, zafirlukast come, è un antagonista del recettore dei leucotrieni utilizzato come alternativa ai farmaci anti-infiammatori nella gestione e nel trattamento cronico dell'asma e di esercizio broncospasmo indotto (BEI). A differenza di zafirlukast, montelukast non inibisce il CYP2C9 o CYP3A4 ed è, quindi, non dovrebbe influenzare la clearance epatica dei farmaci metabolizzati da questi enzimi.
Montelukast Assorbimento
Rapidamente assorbito dopo somministrazione orale (biodisponibilità è del 64%)
Montelukast Tossicita
Gli effetti collaterali includono mal di testa, dolore addominale o mal di stomaco, tosse, mal di denti, vertigini, febbre, bruciore di stomaco, eruzioni cutanee, naso chiuso, debolezza o stanchezza inusuale.
Montelukast Informazioni paziente
General
- Patients should be advised to take montelukast daily as prescribed, even when they are asymptomatic, as well as during periods of worsening asthma, and to contact their physicians if their asthma is not well controlled.
- Patients should be advised that oral tablets of montelukast are not for the treatment of acute asthma attacks. They should have appropriate short-acting inhaled b-agonist medication available to treat asthma exacerbations.
- Patients should be advised that, while using montelukast, medical attention should be sought if short-acting inhaled bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for 24-hour period are needed.
- Patients receiving montelukast should be instructed not to decrease the dose or stop taking any other antiasthma medications unless instructed by a physician.
- Patients who have exacerbations of asthma after exercise should be instructed to continue to use their usual regimen of inhaled b-agonists as prophylaxis unless otherwise instructed by their physician. All patients should have available for rescue a short-acting inhaled b-agonist.
- Patients with known aspirin sensitivity should be advised to continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Phenylketonurics: Phenylketonuric patients should be informed that the chewable tablet contains phenylalanine (a component of aspartame) 0.842 mg per 5-mg chewable tablet.
Montelukast Atto interessato organismi
Gli esseri umani e altri mammiferi