Kinflocin




Categoria
Kinflocin Marchi, Kinflocin Analoghi
- Akilen
- Baccidal
- Bactocin
- Danoflox
- Effexin
- Exocin
- Exocine
- Flobacin
- Flodemex
- Flotavid
- Flovid
- Floxal
- Floxil
- Floxin
- Floxin Otic
- Floxstat
- Fugacin
- Inoflox
- Kinflocin
- Kinoxacin
- Liflox
- Loxinter
- Marfloxacin
- Medofloxine
- Mergexin
- Novecin
- Nufafloqo
- O-Flox
- Obide
- Occidal
- Ocuflox
- Ofcin
- Oflin
- Oflocee
- Oflocet
- Oflocin
- Oflodal
- Oflodex
- Oflodura
- Oflox
- Ofloxin
- Ofus
- Onexacin
- Operan
- Orocin
- Otonil
- Pharflox
- Praxin
- Puiritol
- Qinolon
- Qipro
- Quinolon
- Quotavil
- Rilox
- Sinflo
- Tabrin
- Taravid
- Tariflox
- Tarivid
- Tarivid Eye Ear
- Tarivid Otic
- Telbit
- Tructum
- Uro Tarivid
- Viotisone
- Zanocin
Kinflocin Marchi miscela
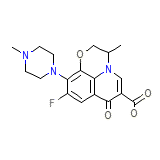
Kinflocin Formula chimica
C18H20FN3O4
Kinflocin RX link
http://www.rxlist.com/cgi/generic/oflox.htm
Kinflocin FDA foglio
Kinflocin DMS (foglio di materiale di sicurezza)
Kinflocin Sintesi di riferimento
I. Hayakava et al. US Pat. 4,382,892 (1983)
Kinflocin Peso molecolare
361.368 g/mol
Kinflocin Temperatura di fusione
250-257 oC
Kinflocin H2O Solubilita
28,3 mg / ml
Kinflocin Stato
Solid
Kinflocin LogP
1.268
Kinflocin Forme di dosaggio
Soluzione (IV iniezione); Tablet; gocce oftalmiche
Kinflocin Indicazione
Per il trattamento di infezioni (tratto respiratorio, dei reni, pelle, tessuti molli, UTI), uretrale e cervicale gonorrea.
Kinflocin Farmacologia
Ofloxacina è un chinolonico / fluorochinolone antibiotico. Ofloxacin è battericida e il suo modo di agire dipende il blocco della replicazione del DNA batterico da legarsi ad un enzima chiamato DNA girasi, che permette al untwisting necessario per replicare una doppia elica del DNA in due. In particolare il farmaco ha affinità 100 volte superiore per DNA girasi batterica rispetto a mammiferi. Ofloxacina è un ampio spettro antibiotico attivo contro i batteri sia Gram-positivi e Gram-negativi.
Kinflocin Assorbimento
Biodisponibilità di ofloxacina nella formulazione in compresse è circa del 98%
Kinflocin Tossicita
LD50 = 5450 mg / kg (per via orale nei topi)
Kinflocin Informazioni paziente
PATIENT INFORMATION
Patients should be advised:
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based
antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for
oral solution should not be taken within the two-hour period before or within the two-hour period after
taking ofloxacin (See Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that
patients should know how they react to ofloxacin before they operate an automobile or machinery or
engage in activities requiring mental alertness and coordination
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture
of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture
has been confidently excluded;
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to
discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat,
difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips,
tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
- to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue
therapy if phototoxicity (e. g. , skin eruption) occurs;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue
ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their
physician before taking this drug if there is a history of this condition.
Patients should be advised:
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based
antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for
oral solution should not be taken within the two-hour period before or within the two-hour period after
taking ofloxacin (See Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that
patients should know how they react to ofloxacin before they operate an automobile or machinery or
engage in activities requiring mental alertness and coordination
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture
of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture
has been confidently excluded;
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to
discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat,
difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips,
tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
- to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue
therapy if phototoxicity (e. g. , skin eruption) occurs;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue
ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their
physician before taking this drug if there is a history of this condition.
Kinflocin Atto interessato organismi
Batteri enterici e altri eubatteri