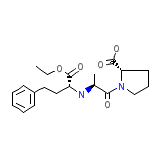
Enalapril




Categoria
Enalapril Marchi, Enalapril Analoghi
Enalapril Marchi miscela
Enalapril Formula chimica
Enalapril RX link
Enalapril FDA foglio
Enalapril DMS (foglio di materiale di sicurezza)
Enalapril Sintesi di riferimento
Enalapril Peso molecolare
Enalapril Temperatura di fusione
Enalapril H2O Solubilita
Enalapril Stato
Enalapril LogP
Enalapril Forme di dosaggio
Enalapril Indicazione
Enalapril Farmacologia
Enalapril Assorbimento
Enalapril Tossicita
Enalapril Informazioni paziente
Angioedema: Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin converting enzyme inhibitors, including enalapril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Hypotension: Patients should be cautioned to report lightheadedness, especially during the first few days of therapy. If actual syncope occurs, the patients should be told to discontinue the drug until they have consulted with the prescribing physician.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with the physician.
Hyperkalemia: Patients should be told not to use salt substitutes containing potassium without consulting their physician. Neutropenia: Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a sign of neutropenia.
Pregnancy: Female patients of childbearing age should be told about the consequences of second- and third-trimester exposure to ACE inhibitors, and they should also be told that these consequences do not appear to have resulted from intrauterine ACEinhibitor exposure that has been limited to the first trimester. These patients should be asked to report pregnancies to their physicians as soon as possible.
NOTE: As with many other drugs, certain advice to patients being treated with enalapril is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.