Xeloda




Categorie
Xeloda Les marques, Xeloda Analogs
Xeloda Les marques melange
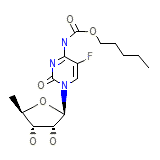
Xeloda Formule chimique
Xeloda RX lien
Xeloda FDA fiche
Xeloda msds (fiche de securite des materiaux)
Xeloda Synthese de reference
Xeloda Poids moleculaire
Xeloda Point de fusion
Xeloda H2O Solubilite
Xeloda Etat
Xeloda LogP
Xeloda Formes pharmaceutiques
Xeloda Indication
Xeloda Pharmacologie
Xeloda Absorption
Xeloda Toxicite
Xeloda Information pour les patients
Patients and patients' caregivers should be informed of the expected adverse effects of XELODA, particularly nausea, vomiting, diarrhea, and hand- and- foot syndrome, and should be made aware that patient- specific dose adaptations during therapy are expected and necessary. Patients should be encouraged to recognize the common grade 2 toxicities associated with XELODA treatment.
Diarrhea: Patients experiencing grade 2 diarrhea (an increase of 4 to 6 stools/day or nocturnal stools) or greater should be instructed to stop taking XELODA immediately. Standard antidiarrheal treatments (e.g., loperamide) are recommended.
Nausea: Patients experiencing grade 2 nausea (food intake significantly decreased but able to eat intermittently) or greater should be instructed to stop taking XELODA immediately. Initiation of symptomatic treatment is recommended.
Vomiting: Patients experiencing grade 2 vomiting (2 to 5 episodes in a 24-hour period) or greater should be instructed to stop taking XELODA immediately. Initiation of symptomatic treatment is recommended.
Hand-and-Foot Syndrome: Patients experiencing grade 2 hand-and-foot syndrome (painful erythema and swelling of the hands and/or feet and/or discomfort affecting the patients' activities of daily living) or greater should be instructed to stop taking XELODA immediately.
Stomatitis: Patients experiencing grade 2 stomatitis (painful erythema, edema or ulcers of the mouth or tongue, but able to eat) or greater should be instructed to stop taking XELODA immediately. Initiation of symptomatic treatment is recommended.
Fever and Neutropenia: Patients who develop a fever of 100.5° F or greater or other evidence of potential infection should be instructed to call their physician.
PATIENT PACKAGE INSERT
This information will help you learn more about XELODA® (capecitabine) Tablets. It cannot, however, cover all possible precautions or side effects associated with XELODA nor does it list all the benefits and risks of XELODA. Your doctor should always be your first choice for detailed information about your medical condition and your treatment. Be sure to ask your doctor about any questions you may have.
What is XELODA?
•XELODA [zeh-LOE-duh] is an oral medication for the treatment of advanced breast cancer resistant to treatment with paclitaxel [pak-lih-TAK-sil] and an anthracycline [ann-thruh-SYE-kleen] containing chemotherapy regimen. Paclitaxel is also known as Taxol®*. Anthracyclines include Adriamycin® or doxorubicin.
XELODA tablets come in two strengths: 150 mg (light peach) and 500 mg (peach).
How does XELODA work?
XELODA is converted in the body to the substance 5-fluorouracil. In some patients, this substance kills cancer cells and decreases the size of the tumor.
Who should not take XELODA?
• Patients allergic to 5-fluorouracil.
• Studies in animals suggest that XELODA may cause serious harm to an unborn child. No studies have been done with pregnant women. If you are pregnant, be sure to discuss with your doctor whether XELODA is right for you. Also, tell your doctor if you are nursing.
How should I take XELODA?
Your doctor will prescribe a dose and treatment regimen that is right for you. Your doctor may want you to take a combination of 150 mg and 500 mg tablets for each dose. If a combination of tablets is prescribed, it is very important that you correctly identify the tablets. Taking the wrong tablets could result in an overdose (too much medication) or underdose (too little medication). The 150 mg tablets are light peach in color and have 150 engraved on one side. The 500 mg tablets are peach in color and have 500 engraved on one side.
• Take the tablets in the combination prescribed by your doctor for your morning and evening doses.
• Take the tablets within 30 minutes after the end of a meal (breakfast and dinner).
• XELODA tablets should be swallowed with water.
• It is important that you take all your medication as prescribed by your doctor.
• If you are taking the vitamin folic acid, please inform your doctor.
• If you are taking warfarin (also known as Coumadin®), please inform your doctor. Your doctor may need to more frequently check how quickly your blood is clotting.
How long will I have to take XELODA?
It is recommended that XELODA be taken for 14 days followed by a 7-day rest period (no drug) given as a 21-day cycle. Your doctor will determine how many cycles of treatment you will need.
What if I miss a dose?
If you miss a dose of XELODA, do not take the missed dose at all and do not double the next one. Instead, continue your regular dosing schedule and check with your doctor.
What are the most common side effects of XELODA?
The most common side effects of XELODA are:
• diarrhea, nausea, vomiting, stomatitis (sores in mouth and throat), abdominal pain, constipation, loss of appetite or decreased appetite, and dehydration (excessive water loss from the body).
• hand-and-foot syndrome (palms of the hands or soles of the feet tingle, become numb, painful, swollen or red), rash, dry or itchy skin.
• tiredness, weakness, dizziness, headache, and fever.
When should I call my doctor?
It is important that you CONTACT YOUR DOCTOR IMMEDIATELY if you experience the following side effects. This will help reduce the likelihood that the side effect will continue or become serious. Your doctor may instruct you to decrease the dose and/or temporarily discontinue treatment with XELODA.
STOP taking XELODA immediately and contact your doctor if any of these symptoms occur:
• Diarrhea: if you have more than 4 bowel movements each day or any diarrhea at night.
• Vomiting: if you vomit more than once in a 24-hour time period.
• Nausea: if you lose your appetite, and the amount of food you eat each day is much less than usual.
• Stomatitis: if you have pain, redness, swelling, or sores in your mouth.
• Hand-and-foot syndrome: if you have pain, swelling, or redness of hands and/or feet.
• Fever or Infection: if you have a temperature of 100.5° F or greater, or other evidence of infection.
If caught early, most of these side effects usually improve within 2 to 3 days after you stop taking XELODA. If they don't improve within 2 to 3 days, call your doctor again. After side effects have improved, your doctor will tell you whether to start taking XELODA again or what dose to use.
How should I store and use XELODA?
• Never share XELODA with anyone.
• XELODA should be stored at normal room temperature (about 65° to 85° F).
• Keep this and all other medications out of the reach of children.
• In case of accidental ingestion or if you suspect that more than the prescribed dose of this medication has been taken, contact your doctor or local poison control center or emergency room IMMEDIATELY.
• Medicines are sometimes prescribed for uses other than those listed in this leaflet. If you have any questions or concerns, or want more information about XELODA, contact your doctor or pharmacist.
* Taxol is a registered trademark of Bristol-Myers Squibb Company.