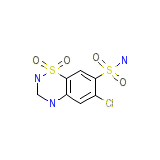
Lopressor HCT




Categorie
Lopressor HCT Les marques, Lopressor HCT Analogs
- Acuretic
- Aldactazide
- Aldoril
- Apresazide
- Aquarills
- Aquarius
- Bremil
- Caplaril
- Capozide
- Chlorosulthiadil
- Chlorothiazide
- Chlorsulfonamidodihydrobenzothiadiazine Dioxide
- Chlorzide
- Cidrex
- Dichlorosal
- Dichlorotride
- Dichlotiazid
- Dichlotride
- Diclotride
- Dicyclotride
- Dihydrochlorothiazid
- Dihydrochlorothiazide
- Dihydrochlorothiazidum
- Dihydrochlorurit
- Dihydrochlorurite
- Dihydroxychlorothiazidum
- Direma
- Disalunil
- Diu-Melusin
- Diuril
- Drenol
- Dyazide
- Esidrex
- Esidrix
- Esimil
- Fluvin
- HCTZ
- HCZ
- Hidril
- Hidrochlortiazid
- Hidroronol
- Hidrotiazida
- Hydril
- Hydro-Aquil
- Hydro-D
- Hydro-Diuril
- Hydrochlorothiazid
- Hydrochlorothiazide Intensol
- Hydrochlorthiazide
- Hydrodiuretic
- Hydrodiuril
- Hydropres
- Hydrosaluric
- Hydrothide
- Hydrozide
- Hypothiazid
- Hypothiazide
- Hyzaar
- Idrotiazide
- Inderide
- Ivaugan
- Jen-Diril
- Lopressor Hct
- Lotensin Hct
- Maschitt
- Maxzide
- Megadiuril
- Microzide
- Moduretic
- Nefrix
- Neo-Codema
- Neoflumen
- Newtolide
- Oretic
- Palonyl
- Panurin
- Perovex
- Primogyn
- Prinzide
- Ro-Hydrazide
- Servithiazid
- Thiaretic
- Thiuretic
- Thlaretic
- Timolide
- Unipres
- Urodiazin
- Vaseretic
- Vetidrex
- Ziac
- Zide
Lopressor HCT Les marques melange
Lopressor HCT Formule chimique
C7H8ClN3O4S2
Lopressor HCT RX lien
http://www.rxlist.com/cgi/generic/hctz.htm
Lopressor HCT FDA fiche
Lopressor HCT msds (fiche de securite des materiaux)
Lopressor HCT Synthese de reference
Werner et al., J. Am. Chem. Soc. 82, 1161 (1960)
Lopressor HCT Poids moleculaire
297.741 g/mol
Lopressor HCT Point de fusion
274 oC
Lopressor HCT H2O Solubilite
0,7 mg / ml
Lopressor HCT Etat
Solid
Lopressor HCT LogP
-0.268
Lopressor HCT Formes pharmaceutiques
Tablet (oral)
Lopressor HCT Indication
Pour le traitement de l'hypertension artérielle et de la gestion de l'œdème.
Lopressor HCT Pharmacologie
Les diurétiques thiazidiques comme l'hydrochlorothiazide favoriser la perte de l'eau du corps (diurétiques). Ils inhibent Na + / Cl-réabsorption du tubule contourné distal dans les reins. Les diurétiques thiazidiques également causer la perte de potassium et une augmentation de l'acide urique sérique. Les diurétiques thiazidiques sont souvent utilisés pour traiter l'hypertension, mais leurs effets hypotenseurs ne sont pas nécessairement en raison de leur activité diurétique. Les diurétiques thiazidiques ont été montré pour empêcher l'hypertension, la morbidité et la mortalité, bien que le mécanisme n'est pas entièrement comprise. Les diurétiques thiazidiques provoquer une vasodilatation en activant activés par le calcium canaux potassiques (grande conductance) dans vasculaires les muscles lisses et en inhibant diverses anhydrases carbonique dans les tissus vasculaires.
Lopressor HCT Absorption
50-60%
Lopressor HCT Toxicite
Les signes les plus communs et les symptômes observés sont ceux causés par la déplétion électrolytique (hypokaliémie, hypochlorémie, hyponatrémie) et la déshydratation résultant d'une diurèse excessive. Si la digitale a également été administrée, l'hypokaliémie peut accentuer les arythmies cardiaques. La DL50 par voie orale, l'hydrochlorothiazide est supérieure à 10 g / kg chez la souris et le rat.
Lopressor HCT Information pour les patients
General
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
Lopressor HCT Organismes affectes
Les humains et autres mammifères