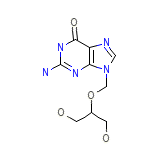
Ganciclovir




Categorie
Ganciclovir Les marques, Ganciclovir Analogs
Ganciclovir Les marques melange
Ganciclovir Formule chimique
C9H13N5O4
Ganciclovir RX lien
http://www.rxlist.com/cgi/generic2/vitrasert.htm
Ganciclovir FDA fiche
Ganciclovir msds (fiche de securite des materiaux)
Ganciclovir Synthese de reference
Alhede, Boerge; et al;. J.Org.Chem;. 56; 6; 2139-2143 (1991)
Ganciclovir Poids moleculaire
255.231 g/mol
Ganciclovir Point de fusion
250 oC
Ganciclovir H2O Solubilite
4,3 mg / ml
Ganciclovir Etat
Solid
Ganciclovir LogP
-1.438
Ganciclovir Formes pharmaceutiques
Insérez occulaire; capsule orale; Injection
Ganciclovir Indication
Pour l'induction et la maintenance dans le traitement des infections à cytomégalovirus (CMV) chez les patients immunodéprimés, notamment les patients atteints du syndrome d'immunodéficience acquise (sida). Également utilisé dans le traitement du cytomégalovirus (CMV) maladie grave, dont la pneumonie à CMV, le CMV maladies gastro-intestinales, les infections à CMV et diffusées, chez les patients immunodéprimés.
Ganciclovir Pharmacologie
Le ganciclovir est un analogue nucléosidique synthétique de la 2'-désoxyguanosine qui inhibe la réplication du virus de l'herpès à la fois in vitro et in vivo. Les virus humains sensibles comprennent le cytomégalovirus (CMV), l'herpès -1 et -2 simplex virus (HSV-1, HSV-2), virus Epstein-Barr (EBV) et le virus varicelle zona (VZV), cependant les études cliniques ont été limitées à l'évaluation de l'efficacité chez les patients avec une infection à CMV. Le ganciclovir est une prodrogue qui est structurellement similaire à l'acyclovir. Il inhibe la réplication du virus par son encorporation dans l'ADN viral. Cette encorporation inhibe dATP et conduit à l'ADN défectueux, cessant ou en retardant la machinerie virale nécessaire pour propager le virus à d'autres cellules.
Ganciclovir Absorption
Faiblement absorbé par voie systémique après administration orale. Biodisponibilité à jeun est d'environ 5%, et quand il est administré avec de la nourriture, de 6 à 9% (environ 30% avec un repas gras).
Ganciclovir Toxicite
Oral, DL50 souris:> 2g/kg. Intraveineuse, chien DL50: 150mg/kg>. Les symptômes de surdose comprennent une pancytopénie irréversible, une aggravation des symptômes gastro-intestinaux, et une insuffisance rénale aiguë. Soupçonné agent anticancéreux.
Ganciclovir Information pour les patients
All patients should be informed that the major toxicities of ganciclovir are granulocytopenia
(neutropenia), anemia and thrombocytopenia and that dose modifications may be required, including
discontinuation. The importance of close monitoring of blood counts while on therapy should be emphasized.
Patients should be informed that ganciclovir has been associated with elevations in serum creatinine.
Patients should be instructed to take CYTOVENE capsules with food to maximize bioavailability.
Patients should be advised that ganciclovir has caused decreased sperm production in animals and may cause
infertility in humans. Women of childbearing potential should be advised that ganciclovir causes birth
defects in animals and should not be used during pregnancy. Women of childbearing potential should be
advised to use effective contraception during treatment with CYTOVENE-IV or CYTOVENE. Similarly, men
should be advised to practice barrier contraception during and for at least 90 days following treatment
with CYTOVENE-IV or CYTOVENE.
Patients should be advised that ganciclovir causes tumors in animals. Although there is no information
from human studies, ganciclovir should be considered a potential carcinogen.
All HIV+ Patients: These patients may be receiving zidovudine (Retrovir?*). Patients should be counseled
that treatment with both ganciclovir and zidovudine simultaneously may not be tolerated by some patients
and may result in severe granulocytopenia (neutropenia). Patients with AIDS may be receiving didanosine
(Videx?#). Patients should be counseled that concomitant treatment with both ganciclovir and didanosine
can cause didanosine serum concentrations to be significantly increased.
HIV+ Patients With CMV Retinitis: Ganciclovir is not a cure for CMV retinitis, and immunocompromised
patients may continue to experience progression of retinitis during or following treatment. Patients
should be advised to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while
being treated with CYTOVENE-IV or CYTOVENE. Some patients will require more frequent follow-up.
Transplant Recipients: Transplant recipients should be counseled regarding the high frequency of impaired
renal function in transplant recipients who received CYTOVENE-IV solution in controlled clinical trials,
particularly in patients receiving concomitant administration of nephrotoxic agents such as cyclosporine
and amphotericin B. Although the specific mechanism of this toxicity, which in most cases was reversible,
has not been determined, the higher rate of renal impairment in patients receiving CYTOVENE-IV solution
compared with those who received placebo in the same trials may indicate that CYTOVENE-IV played a
significant role.
(neutropenia), anemia and thrombocytopenia and that dose modifications may be required, including
discontinuation. The importance of close monitoring of blood counts while on therapy should be emphasized.
Patients should be informed that ganciclovir has been associated with elevations in serum creatinine.
Patients should be instructed to take CYTOVENE capsules with food to maximize bioavailability.
Patients should be advised that ganciclovir has caused decreased sperm production in animals and may cause
infertility in humans. Women of childbearing potential should be advised that ganciclovir causes birth
defects in animals and should not be used during pregnancy. Women of childbearing potential should be
advised to use effective contraception during treatment with CYTOVENE-IV or CYTOVENE. Similarly, men
should be advised to practice barrier contraception during and for at least 90 days following treatment
with CYTOVENE-IV or CYTOVENE.
Patients should be advised that ganciclovir causes tumors in animals. Although there is no information
from human studies, ganciclovir should be considered a potential carcinogen.
All HIV+ Patients: These patients may be receiving zidovudine (Retrovir?*). Patients should be counseled
that treatment with both ganciclovir and zidovudine simultaneously may not be tolerated by some patients
and may result in severe granulocytopenia (neutropenia). Patients with AIDS may be receiving didanosine
(Videx?#). Patients should be counseled that concomitant treatment with both ganciclovir and didanosine
can cause didanosine serum concentrations to be significantly increased.
HIV+ Patients With CMV Retinitis: Ganciclovir is not a cure for CMV retinitis, and immunocompromised
patients may continue to experience progression of retinitis during or following treatment. Patients
should be advised to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while
being treated with CYTOVENE-IV or CYTOVENE. Some patients will require more frequent follow-up.
Transplant Recipients: Transplant recipients should be counseled regarding the high frequency of impaired
renal function in transplant recipients who received CYTOVENE-IV solution in controlled clinical trials,
particularly in patients receiving concomitant administration of nephrotoxic agents such as cyclosporine
and amphotericin B. Although the specific mechanism of this toxicity, which in most cases was reversible,
has not been determined, the higher rate of renal impairment in patients receiving CYTOVENE-IV solution
compared with those who received placebo in the same trials may indicate that CYTOVENE-IV played a
significant role.
Ganciclovir Organismes affectes
Le virus herpétique humain