Apo-Zidovudine




Categorie
Apo-Zidovudine Les marques, Apo-Zidovudine Analogs
Apo-Zidovudine Les marques melange
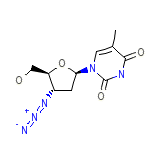
Apo-Zidovudine Formule chimique
Apo-Zidovudine RX lien
Apo-Zidovudine FDA fiche
Apo-Zidovudine msds (fiche de securite des materiaux)
Apo-Zidovudine Synthese de reference
Apo-Zidovudine Poids moleculaire
Apo-Zidovudine Point de fusion
Apo-Zidovudine H2O Solubilite
Apo-Zidovudine Etat
Apo-Zidovudine LogP
Apo-Zidovudine Formes pharmaceutiques
Apo-Zidovudine Indication
Apo-Zidovudine Pharmacologie
Apo-Zidovudine Absorption
Apo-Zidovudine Toxicite
Apo-Zidovudine Information pour les patients
RETROVIR is not a cure for HIV infection, and patients may continue to acquire illnesses associated with HIV infection, including opportunistic infections. Therefore, patients should be advised to seek medical care for any significant change in their health status.
The safety and efficacy of RETROVIR in women, intravenous drug users, and racial minorities is not significantly different than that observed in white males.
Patients should be informed that the major toxicities of RETROVIR are neutropenia and/or anemia. The frequency and severity of these toxicities are greater in patients with more advanced disease and in those who initiate therapy later in the course of their infection. They should be told that if toxicity develops, they may require transfusions or drug discontinuation. They should be told of the extreme importance of having their blood counts followed closely while on therapy, especially for patients with advanced symptomatic HIV disease. They should be cautioned about the use of other medications, including ganciclovir and interferon-alpha, that may exacerbate the toxicity of RETROVIR. Patients should be informed that other adverse effects of RETROVIR include nausea and vomiting. Patients should also be encouraged to contact their physician if they experience muscle weakness, shortness of breath, symptoms of hepatitis or pancreatitis, or any other unexpected adverse events while being treated with RETROVIR.
RETROVIR Tablets, Capsules, and Syrup are for oral ingestion only. Patients should be told of the importance of taking RETROVIR exactly as prescribed. They should be told not to share medication and not to exceed the recommended dose. Patients should be told that the long-term effects of RETROVIR are unknown at this time.
Pregnant women considering the use of RETROVIR during pregnancy for prevention of HIV-transmission to their infants should be advised that transmission may still occur in some cases despite therapy. The long-term consequences of in utero and infant exposure to RETROVIR are unknown, including the possible risk of cancer.
HIV-infected pregnant women should be advised not to breastfeed to avoid postnatal transmission of HIV to a child who may not yet be infected.
Patients should be advised that therapy with RETROVIR has not been shown to reduce the risk of transmission of HIV to others through sexual contact or blood contamination.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.