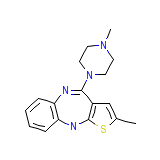
Zydis




Categoria
Zydis Nombres de marca, Zydis Analogos
Zydis Marca los nombres de mezcla
Zydis Formula quimica
C17H20N4S
Zydis RX enlace
http://www.rxlist.com/cgi/generic3/symbyax.htm
Zydis FDA hoja
Zydis MSDS (hoja de seguridad de materiales)
Zydis Sintesis de referencia
JK Chakrabarti et al., Patente de EE.UU.. 5,229,382 (1991)
Zydis Peso molecular
312.434 g/mol
Zydis Punto de fusion
195oC
Zydis H2O Solubilidad
No hay información disponible
Zydis Estado
Solid
Zydis LogP
2.199
Zydis Formas de dosificacion
Tablet (oral)
Zydis Indicacion
Para el tratamiento de la esquizofrenia y el trastorno maníaco depresivo (trastorno bipolar).
Zydis Farmacologia
La olanzapina, un antipsicótico atípico, se usa para tratar tanto los síntomas positivos y negativos de la esquizofrenia, manía aguda con trastorno bipolar, la agitación y los síntomas psicóticos en la demencia. Futuro usos pueden incluir el tratamiento de trastorno obsesivo-compulsivo y trastornos graves de conducta en el autismo. Estructural y farmacológicamente similar a la clozapina, la olanzapina se une a la alfa (1), la dopamina, , La histamina H1, muscarínicos, y la serotonina tipo 2 (5-HT 2) receptores.
Zydis Absorcion
Se absorbe bien, aproximadamente el 40% de la dosis metabolizada antes de alcanzar la circulación sistémica.
Zydis Toxicidad
No hay información disponible
Zydis Informacion de Pacientes
PATIENT INFORMATION
Physicians are advised to discuss the following issues with patients for whom they prescribe
olanzapine:
Orthostatic Hypotension: Patients should be advised of the risk of orthostatic hypotension,
especially during the period of initial dose titration and in association with the use of
concomitant drugs that may potentiate the orthostatic effect of olanzapine, e.g., diazepam
or alcohol.
Interference with Cognitive and Motor Performance: Because olanzapine has the potential to
impair judgment, thinking, or motor skills, patients should be cautioned about operating
hazardous machinery, including automobiles, until they are reasonably certain that olanzapine
therapy does not affect them adversely.
Pregnancy: Patients should be advised to notify their physician if they become pregnant or
intend to become pregnant during therapy with olanzapine.
Nursing: Patients should be advised not to breast-feed an infant if they are taking olanzapine.
Concomitant Medication: Patients should be advised to inform their physicians if they are taking,
or plan to take, any prescription or over-the-counter drugs, since there is a potential for
interactions.
Alcohol: Patients should be advised to avoid alcohol while taking olanzapine.
Heat Exposure and Dehydration: Patients should be advised regarding appropriate care in avoiding
overheating and dehydration.
Phenylketonurics: ZYPREXA ZYDIS (olanzapine orally disintegrating tablets) contains phenylalanine
(0.34, 0.45, 0.67, or 0.90 mg per 5, 10, 15, or 20mg tablet, respectively).
Laboratory Tests
Periodic assessment of transaminases is recommended in patients with significant hepatic disease.
Physicians are advised to discuss the following issues with patients for whom they prescribe
olanzapine:
Orthostatic Hypotension: Patients should be advised of the risk of orthostatic hypotension,
especially during the period of initial dose titration and in association with the use of
concomitant drugs that may potentiate the orthostatic effect of olanzapine, e.g., diazepam
or alcohol.
Interference with Cognitive and Motor Performance: Because olanzapine has the potential to
impair judgment, thinking, or motor skills, patients should be cautioned about operating
hazardous machinery, including automobiles, until they are reasonably certain that olanzapine
therapy does not affect them adversely.
Pregnancy: Patients should be advised to notify their physician if they become pregnant or
intend to become pregnant during therapy with olanzapine.
Nursing: Patients should be advised not to breast-feed an infant if they are taking olanzapine.
Concomitant Medication: Patients should be advised to inform their physicians if they are taking,
or plan to take, any prescription or over-the-counter drugs, since there is a potential for
interactions.
Alcohol: Patients should be advised to avoid alcohol while taking olanzapine.
Heat Exposure and Dehydration: Patients should be advised regarding appropriate care in avoiding
overheating and dehydration.
Phenylketonurics: ZYPREXA ZYDIS (olanzapine orally disintegrating tablets) contains phenylalanine
(0.34, 0.45, 0.67, or 0.90 mg per 5, 10, 15, or 20mg tablet, respectively).
Laboratory Tests
Periodic assessment of transaminases is recommended in patients with significant hepatic disease.
Zydis Organismos afectados
Humanos y otros mamíferos