Theoclair-SR




Categoria
Theoclair-SR Nombres de marca, Theoclair-SR Analogos
- Accurbron
- Acet-Theocin
- Aerobin
- Aerolate
- Aerolate III
- Aerolate SR
- Afonilm
- Afonilum
- Aminophylline
- Aquaphyllin
- Armophylline
- Asbron
- Asmalix
- Asmax
- Austyn
- Bilordyl
- Bronchoretard
- Bronkodyl
- Bronkodyl SR
- Cetraphylline
- Choledyl SA
- Chronophyllin
- Constant T
- Diffumal
- Dimethylxanthine
- Diphyllin
- Doraphyllin
- Duraphyl
- Duraphyllin
- Dyspne-Inhal
- Egifilin
- Elan
- Elixex
- Elixicon
- Elixomin
- Elixophyllin
- Elixophyllin SR
- Elixophylline
- Etheophyl
- Euphyllin
- Euphylline
- Euphylong
- Labid
- Lanophyllin
- Lasma
- Liquophylline
- Liquorice
- Maphylline
- Medaphyllin
- Nuelin
- Optiphyllin
- Parkophyllin
- Physpan
- Pro-Vent
- Pseudotheophylline
- Pulmidur
- Pulmo-Timelets
- Quibron
- Quibron T
- Quibron T/SR
- Quibron-T
- Quibron-T/SR
- Respbid
- Respicur
- Respid
- Slo-Bid
- Slo-Phyllin
- Solosin
- Somophyllin CRT
- Somophyllin T
- Somophyllin-CRT
- Somophyllin-DF
- Somophyllin-T
- Spophyllin Retard
- Sustaire
- Synophylate
- Synophylate-L.A. Cenules
- T-Phyl
- Talotren
- Tefamin
- Telb-DS
- Telbans Dry Syrup
- Teocen 200
- Teofilina
- Teofyllamin
- Teolair
- Teonova
- Teosona
- Tesona
- Theacitin
- Theal Tablets
- Theo 24
- Theo-11
- Theo-24
- Theo-DS
- Theo-Dur
- Theo-Nite
- Theo-Sav
- Theobid
- Theobid Duracap
- Theobid Jr.
- Theochron
- Theocin
- Theoclair-SR
- Theoclear
- Theoclear 80
- Theoclear L.A.-130
- Theoclear LA
- Theoclear-200
- Theoclear-80
- Theocontin
- Theodel
- Theodrip
- Theodur Dry Syrup
- Theofol
- Theograd
- Theolair
- Theolair-SR
- Theolix
- Theolixir
- Theon
- Theona P
- Theopek
- Theophyl
- Theophyl-225
- Theophyl-SR
- Theophyline
- Theophyllin
- Theophylline Anhydrous
- Theophylline, Anhydrous
- Theophylline-SR
- Theoplus
- Theospan
- Theostat
- Theostat 80
- Theotard
- Theovent
- Uni-Dur
- Unifyl
- Unilong
- Uniphyl
- Uniphyllin
- Xanthium
- Xantivent
Theoclair-SR Marca los nombres de mezcla
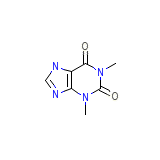
Theoclair-SR Formula quimica
Theoclair-SR RX enlace
Theoclair-SR FDA hoja
Theoclair-SR MSDS (hoja de seguridad de materiales)
Theoclair-SR Sintesis de referencia
Theoclair-SR Peso molecular
Theoclair-SR Punto de fusion
Theoclair-SR H2O Solubilidad
Theoclair-SR Estado
Theoclair-SR LogP
Theoclair-SR Formas de dosificacion
Theoclair-SR Indicacion
Theoclair-SR Farmacologia
Theoclair-SR Absorcion
Theoclair-SR Toxicidad
Theoclair-SR Informacion de Pacientes
Immediate Release Products
The patient (or parent/care giver) should be instructed to seek medical advice whenever nausea, vomiting, persistent headache, insomnia or rapid heart beat occurs during treatment with theophylline, even if another cause is suspected. The patient should be instructed to contact their clinician if they develop a new illness, especially if accompanied by a persistent fever, if they experience worsening of a chronic illness, if they start or stop smoking cigarettes or marijuana, or if another clinician and a new medication or discontinues a previously prescribed medication. Patients should be instructed to inform all clinicians involved in their care that they are taking theophylline, especially when a medication is being added or deleted from their treatment. Patients should be instructed to not alter the dose, timing of the dose, or frequency of administration without first consulting their clinician. If a dose is missed, the patient should be instructed to take the next dose at the usually scheduled time and to not attempt to make up for the missed dose.
Extended-Release Capsules
This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
The physician should reinforce the importance of taking only the prescribed dose and the time interval between doses. As with any controlled-release theophylline product, the patient should alert the physician of symptoms occur repeatedly, especially near the end of the dosing interval.
When prescribing administration by the sprinkle method, details of the proper technique should be explained to patient
Patients should be informed of the need to take this drug in the fasting state, and that drug administration should be 1 hour before or 2 hours after meals.