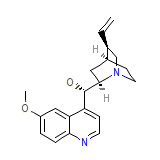
Quinidine Gluconate




Categoria
Quinidine Gluconate Nombres de marca, Quinidine Gluconate Analogos
- Apo-Quinidine
- Biquin Durules
- Cardioquin
- Chinidin
- Cin-Quin
- Coccinine
- Conchinin
- Conchinine
- Conquinine
- Duraquin
- Kinidin
- Novoquinidin
- Pitayin
- Pitayine
- Quin-Release
- Quinact
- Quinaglute
- Quinaglute Dura-Tabs
- Quinalan
- Quinate
- Quinatime
- Quindine
- Quinicardine
- Quinidex
- Quinidex Extentabs
- Quinidine Gluconate
- Quinidine Sulfate
- Quinine, Polymers
- Quinora
Quinidine Gluconate Marca los nombres de mezcla
Quinidine Gluconate Formula quimica
C20H24N2O2
Quinidine Gluconate RX enlace
http://www.rxlist.com/cgi/generic/quinidine.htm
Quinidine Gluconate FDA hoja
Quinidine Gluconate MSDS (hoja de seguridad de materiales)
Quinidine Gluconate Sintesis de referencia
No hay información disponible
Quinidine Gluconate Peso molecular
324.417 g/mol
Quinidine Gluconate Punto de fusion
174 oC
Quinidine Gluconate H2O Solubilidad
140 mg / L
Quinidine Gluconate Estado
Solid
Quinidine Gluconate LogP
2.534
Quinidine Gluconate Formas de dosificacion
Solución
Quinidine Gluconate Indicacion
Para el tratamiento de pre-excitación ventricular y arritmias cardíacas
Quinidine Gluconate Farmacologia
Quinidina, un anticonvulsivo fenitoína, se usa solo o junto con fenobarbital u otros anticonvulsivos para gestionar crisis tónico-clónicas, ataques psicomotores, los síndromes de dolor neuropático diabético incluyendo neuropatía, inducidas por digitálicos arritmias cardíacas y arritmias cardíacas asociadas con prolongación del intervalo QT.
Quinidine Gluconate Absorcion
No hay información disponible
Quinidine Gluconate Toxicidad
No hay información disponible
Quinidine Gluconate Informacion de Pacientes
Before prescribing QUINAGLUTE® as prophylaxis against recurrence of atrial fibrillation, the physician should inform the patient of the risks and benefits to be expected. Discussion should include the facts
- that the goal of therapy will be a reduction (probably not to zero) in the frequency of episodes of atrial fibrillation; and
- that reduced frequency of fibrillatory episodes may be expected, if achieved, to bring symptomatic benefit; but
- that no data are available to show that reduced frequency of fibrillatory episodes will reduce the risks of irreversible harm through stroke or death; and in fact
- that such data as are available suggest that treatment with QUINAGLUTE® is likely to increase the patient's risk of death.
To confirm whether this is the most current prescribing information available on Quinaglute®, or to obtain the most current prescribing information, please call Berlex Laboratories at 1-888-BERLEX-4 (choose option #4, Product Usage Information).
Quinidine Gluconate Organismos afectados
Humanos y otros mamíferos