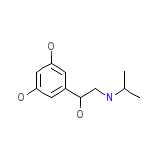
Prometa




Categoria
Prometa Nombres de marca, Prometa Analogos
Prometa Marca los nombres de mezcla
Prometa Formula quimica
C11H17NO3
Prometa RX enlace
http://www.rxlist.com/cgi/generic2/metaproterenol.htm
Prometa FDA hoja
Prometa MSDS (hoja de seguridad de materiales)
Prometa Sintesis de referencia
O. Thoma, K. Zeile, EE.UU. Pat. 3,341,594 (1967)
Prometa Peso molecular
211.258 g/mol
Prometa Punto de fusion
100oC
Prometa H2O Solubilidad
9.7mg / L
Prometa Estado
Solid
Prometa LogP
1.172
Prometa Formas de dosificacion
Inhalación
Prometa Indicacion
Para el tratamiento del broncoespasmo, bronquitis crónica, asma y enfisema.
Prometa Farmacologia
Metaproterenol, una amina sintética, es estructural y farmacológicamente similar al isoproterenol. Metaproterenol se utiliza exclusivamente como un broncodilatador. Los efectos farmacológicos de los agonistas beta-adrenérgicos drogas, tales como metaproterenol, al menos en parte atribuible a la estimulación a través de receptores beta-adrenérgicos de la adenilato ciclasa intracelular, la enzima que cataliza la conversión de la adenosina trifosfato (ATP) para cíclico-3 ', 5'-monofosfato de adenosina (c-AMP). El aumento de AMP-c niveles están asociados con la relajación del músculo liso bronquial y la inhibición de la liberación de mediadores de inmediato hipersensibilidad de las células, especialmente de los mastocitos.
Prometa Absorcion
3% (biodisponibilidad oral del 40%)
Prometa Toxicidad
Los síntomas de sobredosis incluyen la angina de pecho, hipertensión o hipotensión, arritmias, nerviosismo, dolor de cabeza, temblor, sequedad de boca, palpitaciones, náuseas, mareos, fatiga, malestar general y el insomnio. DL50 = 42 mg / kg (por vía oral en ratas).
Prometa Informacion de Pacientes
Patient�s Instructions for Use Alupent�
(metaproterenol sulfate USP) Inhalation Aerosol
1. Insert metal canister into clear end of mouthpiece.
2. Remove protective cap, invert canister and shake well before each use.
3. Avoid spraying in eyes.
4. Enclose mouthpiece with the lips. The base of the canister should be held vertically. The Alupent� canister is to be used only with the white Alupent� Inhalation Aerosol mouthpiece. This mouthpiece should not be used with other aerosol medications.
5. Exhale deeply, then inhale slowly through the mouth and at the same time firmly press once on the upended canister base; continue to inhale deeply. Hold your breath for a few seconds and then remove the mouthpiece from the mouth and exhale slowly.
One inhalation is often enough to obtain relief. The inhalation can be repeated once or twice, if necessary, or as your physician directs. Wait at least two minutes before repeating the inhalation. In most cases, the dose should not be repeated more often than every 3 to 4 hours. No more than 12 inhalations should be taken in one day.
6. Replace protective cap after use.
WARNING
Do not exceed the dose prescribed by your physician. If difficulty in breathing persists, contact your physician immediately.
Note: When full, the container holds enough medication for at least 200 inhalations: at least 100 inhalations are in the sample unit. Check regularly, by shaking the cylinder or container, to determine whether it contains any medication. When it first seems empty, there are still about ten doses left. Refill containers for the plastic mouthpiece are available when prescribed by your physician.
Keep the mouthpiece clean. Wash with hot water. If soap is used, rinse thoroughly with plain water.
Never open the container holding the medication. Opening it is dangerous and renders the contents useless.
Caution: Contents Under Pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120�F. Keep out of reach of small children
Note: The indented statement below is required by the Federal government�s Clean Air Act for all products containing or manufactured with chlorofluorocarbons (CFCs): This product contains trichloromonofluoromethane (CFC-11), dichlorodifluoromethane (CFC-12) and dichlorotetrafluoroethane (CFC-114), substances which harm the environment by destroying ozone in the upper atmosphere.
Your physician has determined that this product is likely to help your personal health. USE THIS PRODUCT AS DIRECTED, UNLESS INSTRUCTED TO DO OTHERWISE BY YOUR PHYSICIAN. If you have any questions about alternatives, consult with your physician
(metaproterenol sulfate USP) Inhalation Aerosol
1. Insert metal canister into clear end of mouthpiece.
2. Remove protective cap, invert canister and shake well before each use.
3. Avoid spraying in eyes.
4. Enclose mouthpiece with the lips. The base of the canister should be held vertically. The Alupent� canister is to be used only with the white Alupent� Inhalation Aerosol mouthpiece. This mouthpiece should not be used with other aerosol medications.
5. Exhale deeply, then inhale slowly through the mouth and at the same time firmly press once on the upended canister base; continue to inhale deeply. Hold your breath for a few seconds and then remove the mouthpiece from the mouth and exhale slowly.
One inhalation is often enough to obtain relief. The inhalation can be repeated once or twice, if necessary, or as your physician directs. Wait at least two minutes before repeating the inhalation. In most cases, the dose should not be repeated more often than every 3 to 4 hours. No more than 12 inhalations should be taken in one day.
6. Replace protective cap after use.
WARNING
Do not exceed the dose prescribed by your physician. If difficulty in breathing persists, contact your physician immediately.
Note: When full, the container holds enough medication for at least 200 inhalations: at least 100 inhalations are in the sample unit. Check regularly, by shaking the cylinder or container, to determine whether it contains any medication. When it first seems empty, there are still about ten doses left. Refill containers for the plastic mouthpiece are available when prescribed by your physician.
Keep the mouthpiece clean. Wash with hot water. If soap is used, rinse thoroughly with plain water.
Never open the container holding the medication. Opening it is dangerous and renders the contents useless.
Caution: Contents Under Pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120�F. Keep out of reach of small children
Note: The indented statement below is required by the Federal government�s Clean Air Act for all products containing or manufactured with chlorofluorocarbons (CFCs): This product contains trichloromonofluoromethane (CFC-11), dichlorodifluoromethane (CFC-12) and dichlorotetrafluoroethane (CFC-114), substances which harm the environment by destroying ozone in the upper atmosphere.
Your physician has determined that this product is likely to help your personal health. USE THIS PRODUCT AS DIRECTED, UNLESS INSTRUCTED TO DO OTHERWISE BY YOUR PHYSICIAN. If you have any questions about alternatives, consult with your physician
Prometa Organismos afectados
Humanos y otros mamíferos