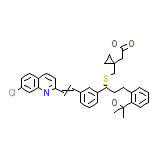
Montelukast




Categoria
Montelukast Nombres de marca, Montelukast Analogos
Montelukast Marca los nombres de mezcla
Montelukast Formula quimica
C35H36ClNO3S
Montelukast RX enlace
http://www.rxlist.com/cgi/generic3/monteluk.htm
Montelukast FDA hoja
Montelukast MSDS (hoja de seguridad de materiales)
Montelukast Sintesis de referencia
ML Belley et al., Eur. Pat. Appl. 480,717 (1992)
Montelukast Peso molecular
586.184 g/mol
Montelukast Punto de fusion
No information avaliable
Montelukast H2O Solubilidad
No hay información disponible
Montelukast Estado
Solid
Montelukast LogP
8.488
Montelukast Formas de dosificacion
Tablet (oral)
Montelukast Indicacion
Para el tratamiento del asma
Montelukast Farmacologia
Montelukast, zafirlukast como, es un antagonista del receptor de leucotrienos utilizado como una alternativa a los medicamentos anti-inflamatorios en la gestión y el tratamiento crónico del asma y broncoespasmo inducido por el ejercicio (BEI). A diferencia de zafirlukast, montelukast no inhibe CYP2C9 o CYP3A4 y, por tanto, no espera que afecte a la eliminación hepática de los fármacos metabolizados por estas enzimas.
Montelukast Absorcion
Absorbe rápidamente tras la administración oral (biodisponibilidad es del 64%)
Montelukast Toxicidad
Los efectos secundarios incluyen dolor de cabeza, dolor abdominal o estomacal, tos, dolor dental, mareos, fiebre, ardor de estómago, erupciones en la piel, congestión nasal, debilidad o cansancio inusual.
Montelukast Informacion de Pacientes
General
- Patients should be advised to take montelukast daily as prescribed, even when they are asymptomatic, as well as during periods of worsening asthma, and to contact their physicians if their asthma is not well controlled.
- Patients should be advised that oral tablets of montelukast are not for the treatment of acute asthma attacks. They should have appropriate short-acting inhaled b-agonist medication available to treat asthma exacerbations.
- Patients should be advised that, while using montelukast, medical attention should be sought if short-acting inhaled bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for 24-hour period are needed.
- Patients receiving montelukast should be instructed not to decrease the dose or stop taking any other antiasthma medications unless instructed by a physician.
- Patients who have exacerbations of asthma after exercise should be instructed to continue to use their usual regimen of inhaled b-agonists as prophylaxis unless otherwise instructed by their physician. All patients should have available for rescue a short-acting inhaled b-agonist.
- Patients with known aspirin sensitivity should be advised to continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Phenylketonurics: Phenylketonuric patients should be informed that the chewable tablet contains phenylalanine (a component of aspartame) 0.842 mg per 5-mg chewable tablet.
Montelukast Organismos afectados
Humanos y otros mamíferos