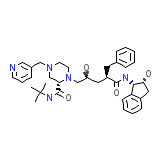
Indinavir




Categoria
Indinavir Nombres de marca, Indinavir Analogos
Indinavir Marca los nombres de mezcla
Indinavir Formula quimica
Indinavir RX enlace
Indinavir FDA hoja
Indinavir MSDS (hoja de seguridad de materiales)
Indinavir Sintesis de referencia
Indinavir Peso molecular
Indinavir Punto de fusion
Indinavir H2O Solubilidad
Indinavir Estado
Indinavir LogP
Indinavir Formas de dosificacion
Indinavir Indicacion
Indinavir Farmacologia
Indinavir Absorcion
Indinavir Toxicidad
Indinavir Informacion de Pacientes
A statement to patients and health care providers is included on the product’s bottle label. ALERT: Find out about medicines that should NOT be taken with CRIXIVAN. A Patient Package Insert (PPI) for CRIXIVAN is available for patient information.
CRIXIVAN is not a cure for HIV infection and patients may continue to develop opportunistic infections and other complications associated with HIV disease. The long-term effects of CRIXIVAN are unknown at this time. CRIXIVAN has not been shown to reduce the risk of transmission of HIV to others through sexual contact or blood contamination.
Patients should be advised to remain under the care of a physician when using CRIXIVAN and should not modify or discontinue treatment without first consulting the physician. Therefore, if a dose is missed, patients should take the next dose at the regularly scheduled time and should not double this dose. Therapy with CRIXIVAN should be initiated and maintained at the recommended dosage.
CRIXIVAN may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John’s wort.
For optimal absorption, CRIXIVAN should be administered without food but with water 1 hour before or 2 hours after a meal. Alternatively, CRIXIVAN may be administered with other liquids such as skim milk, juice, coffee, or tea, or with a light meal, e.g., dry toast with jelly, juice, and coffee with skim milk and sugar; or corn flakes, skim milk and sugar. Ingestion of CRIXIVAN with a meal high in calories, fat, and protein reduces the absorption of indinavir.
Patients receiving a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil, tadalafil, or vardenafil) should be advised that they may be at an increased risk of PDE5 inhibitor-associated adverse events including hypotension, visual changes, and priapism, and should promptly report any symptoms to their doctors.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
CRIXIVAN Capsules are sensitive to moisture. Patients should be informed that CRIXIVAN should be stored and used in the original container and the desiccant should remain in the bottle.