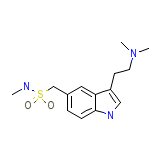
Imitrex




Categoria
Imitrex Nombres de marca, Imitrex Analogos
Imitrex Marca los nombres de mezcla
Imitrex Formula quimica
C14H21N3O2S
Imitrex RX enlace
http://www.rxlist.com/cgi/generic/sumitr.htm
Imitrex FDA hoja
Imitrex MSDS (hoja de seguridad de materiales)
Imitrex Sintesis de referencia
MD Dowle, IH Coates, EE.UU. Pat. 4,816,470 (1989)
Imitrex Peso molecular
295.402 g/mol
Imitrex Punto de fusion
169-171 oC
Imitrex H2O Solubilidad
21,4 mg / ml
Imitrex Estado
Solid
Imitrex LogP
0.965
Imitrex Formas de dosificacion
Tableta, inyección, aerosol nasal
Imitrex Indicacion
Para el tratamiento de ataques de migraña con o sin aura en adultos
Imitrex Farmacologia
Sumatriptán, un fármaco contra la migraña, es estructuralmente similar a la serotonina. Se cree que la constricción de los vasos sanguíneos cerebrales inducida por la activación de receptores 5-HT1 en los buques pueden contribuir al efecto antimigrainous de sumatriptán en humanos.
Imitrex Absorcion
~ 15%
Imitrex Toxicidad
convulsiones, temblores, parálisis, falta de actividad, ptosis, eritema de las extremidades, respiración anormal, cianosis, ataxia, midriasis, salivación, lagrimeo y, LD50 = mg / kg (por vía oral en ratones)
Imitrex Informacion de Pacientes
PATIENT INFORMATION
PATIENT PACKAGE INSERT
Please read this information carefully before you take sumatriptan succinate injection, tablets or nasal spray. This provides a
summary of the information available on your medicine. Please do not throw away this information until you have finished your
medicine. You may need to read this information again. This PATIENT PACKAGE INSERT does not contain all of the information on
sumatriptan succinate injection, tablets, or nasal spray. For further information or advice, ask your doctor or pharmacist.
Information About Your Medicine
The generic name of your medication is sumatriptan succinate. It can be obtained only with a prescription from your doctor. The
decision to use sumatriptan injection, tablets, or nasal spray is one that you and your doctor should make jointly, taking into
account your individual preferences and medical circumstances. If you have risk factors for heart disease (such as high blood
pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male
over 40), you should tell your doctor, who should evaluate you for heart disease in order to determine if sumatriptan succinate is
appropriate for you. Although the vast majority of those who have taken sumatriptan succinate have not experienced any significant
side effects, some individuals have experienced serious heart problems and, rarely, considering the extensiveness of sumatriptan
succinate use worldwide, deaths have been reported. In all but a few instances, however, serious problems occurred in people with
known heart disease and it was not clear whether sumatriptan succinate was a contributory factor in these deaths.
The Purpose of Your Medicine
Injection: Sumatriptan succinate injection is intended to relieve your migraine or cluster headache, but not to prevent or reduce
the number of attacks you experience. Use sumatriptan succinate injection only to treat an actual migraine or cluster headache attack.
Tablets: Sumatriptan succinate tablets are intended to relieve your migraine, but not to prevent or reduce the number of attacks you
experience. Use sumatriptan succinate tablets only to treat an actual migraine attack.
Nasal Spray: Sumatriptan nasal spray is intended to relieve your migraine, but not to prevent or reduce the number of attacks you
experience. Use sumatriptan nasal spray only to treat an actual migraine attack.
Important Questions to Consider Before Taking Sumatriptan
If the answer to any of the following questions is YES or if you do not know the answer, then please discuss with your doctor before
you use sumatriptan succinate injection, tablets, or nasal spray.
Are you pregnant? Do you think you might be pregnant? Are you trying to become pregnant? Are you using inadequate contraception? Are
you breast-feeding?
Do you have any chest pain, heart disease, shortness of breath, or irregular heartbeats? Have you had a heart attack?
Do you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family
history of heart disease, or you are postmenopausal or a male over 40)?
Have you had a stroke, transient ischemic attacks (TIAs) or Raynaud syndrome?
Do you have high blood pressure?
Have you ever had to stop taking this or any other medication because of an allergy or any other problems?
Are you taking any other medications, including other 5-HT1 agonists or any other migraine medications containing ergotamine,
dihydroergotamine, or methysergide.
Are you taking any medication for depression (monoamine oxidase inhibitors or selective serotonin reuptake inhibitors [SSRIs])?
Have you had, or do you have, any disease of the liver or kidney?
Have you had, or do you have, epilepsy or seizures?
Is this headache different from your usual migraine attacks?
Remember, if you answered YES to any of the above questions, then discuss it with your doctor.
The Use of Sumatriptan Succinate During Pregnancy
Do not use sumatriptan succinate injection, tablets, or nasal spray if you are pregnant, think you might be pregnant, are trying to
become pregnant, or are not using adequate contraception, unless you have discussed this with your doctor.
How to Use Sumatriptan Succinate?
Injection: Before injecting sumatriptan succinate, check with your doctor on acceptable injection sites and see the instructions (on
or inside the carton) on discarding empty syringes and loading an autoinjector device.
Never reuse a syringe.
For adults, the usual dose is a single injection given just below the skin. It should be given as soon as the symptoms of your migraine
appear, but may be given at any time during an attack. A second injection may be given if your symptoms of migraine come back. If your
symptoms do not improve following the first injection, do not give a second injection for the same attack without first consulting with
your doctor. Do not have more than two injections in any 24 hours and allow at least 1 hour between each dose.
Tablets: For adults, the usual dose is a single tablet taken whole with fluids. A second tablet may be taken if your symptoms of migraine
come back or if you have a partial response to the initial dose, but not sooner than 2 hours following the first tablet. For a given
attack, if you have no response to the first tablet, do not take a second tablet without first consulting with your doctor. Do not take
more than a total of 200 mg of sumatriptan succinate tablets in any 24-hour period. The safety of treating an average of more than four
headaches in a 30-day period has not been established.
Nasal Spray: Before using sumatriptan nasal spray, see the enclosed instruction pamphlet. For adults, the usual dose is a single nasal
spray administered into one nostril. If your headache comes back, a second nasal spray may be administered anytime after 2 hours of
administering the first spray. For any attack where you have no response to the first nasal spray, do not take a second nasal spray
without first consulting with your doctor. Do not administer more than a total of 40 mg of sumatriptan nasal spray in any 24-hour
period. The effects of long-term repeated use of sumatriptan nasal spray on the surfaces of the nose and throat have not been specifically
studied. The safety of treating an average of more than four headaches in a 30-day period has not been established.
Side Effects to Watch For
Some patients experience pain or tightness in the chest or throat when using sumatriptan succinate injection, tablets, or nasal spray.
If this happens to you, then discuss it with your doctor before using any more sumatriptan succinate. If the chest pain is severe or
does not go away, call your doctor immediately.
If you have sudden and/or severe abdominal pain following sumatriptan succinate, call your doctor immediately.
Shortness of breath; wheeziness; heart throbbing; swelling of eyelids, face, or lips; or a skin rash, skin lumps, or hives happen
rarely. If it happens to you, then tell your doctor immediately. Do not take any more sumatriptan succinate injection, tablets, or
nasal spray unless your doctor tells you to do so.
Some people may have feelings of tingling, heat, flushing (redness of face lasting a short time), heaviness or pressure after
treatment with sumatriptan succinate injection, tablets, or nasal spray. A few people may feel drowsy, dizzy, tired or sick, (or
experience nasal irritation with the nasal spray only). Tell your doctor of these symptoms at your next visit.
If you should feel unwell in any other way or have symptoms that you do not understand, you should contact your doctor immediately.
NOTE for Injection: You may experience pain or redness at site of injection, but this usually lasts less than an hour.
What to do if an Overdose is Taken
If you have taken more medication than you have been told, contact either your doctor, hospital emergency department, or nearest
poison control center immediately.
Storing Your Medicine
Keep your medicine in a safe place where children cannot reach it. It may be harmful to children. Store your medication from heat
and light. Tablets and Nasal Spray: Do not store at temperatures above 30�C (86�F) or below 2�C (36�F).
If your medication has expired (the expiration date is printed on the treatment pack), throw it away as instructed.
If your doctor decides to stop treatment, do not keep any leftover medicine unless your doctor tells you to. Throw away your medicine
as instructed.
For Injection: Do not throw away your autoinjector. Keep your medication in the case provided and do not store at temperatures above
30�C (86�F).
PATIENT PACKAGE INSERT
Please read this information carefully before you take sumatriptan succinate injection, tablets or nasal spray. This provides a
summary of the information available on your medicine. Please do not throw away this information until you have finished your
medicine. You may need to read this information again. This PATIENT PACKAGE INSERT does not contain all of the information on
sumatriptan succinate injection, tablets, or nasal spray. For further information or advice, ask your doctor or pharmacist.
Information About Your Medicine
The generic name of your medication is sumatriptan succinate. It can be obtained only with a prescription from your doctor. The
decision to use sumatriptan injection, tablets, or nasal spray is one that you and your doctor should make jointly, taking into
account your individual preferences and medical circumstances. If you have risk factors for heart disease (such as high blood
pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male
over 40), you should tell your doctor, who should evaluate you for heart disease in order to determine if sumatriptan succinate is
appropriate for you. Although the vast majority of those who have taken sumatriptan succinate have not experienced any significant
side effects, some individuals have experienced serious heart problems and, rarely, considering the extensiveness of sumatriptan
succinate use worldwide, deaths have been reported. In all but a few instances, however, serious problems occurred in people with
known heart disease and it was not clear whether sumatriptan succinate was a contributory factor in these deaths.
The Purpose of Your Medicine
Injection: Sumatriptan succinate injection is intended to relieve your migraine or cluster headache, but not to prevent or reduce
the number of attacks you experience. Use sumatriptan succinate injection only to treat an actual migraine or cluster headache attack.
Tablets: Sumatriptan succinate tablets are intended to relieve your migraine, but not to prevent or reduce the number of attacks you
experience. Use sumatriptan succinate tablets only to treat an actual migraine attack.
Nasal Spray: Sumatriptan nasal spray is intended to relieve your migraine, but not to prevent or reduce the number of attacks you
experience. Use sumatriptan nasal spray only to treat an actual migraine attack.
Important Questions to Consider Before Taking Sumatriptan
If the answer to any of the following questions is YES or if you do not know the answer, then please discuss with your doctor before
you use sumatriptan succinate injection, tablets, or nasal spray.
Are you pregnant? Do you think you might be pregnant? Are you trying to become pregnant? Are you using inadequate contraception? Are
you breast-feeding?
Do you have any chest pain, heart disease, shortness of breath, or irregular heartbeats? Have you had a heart attack?
Do you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family
history of heart disease, or you are postmenopausal or a male over 40)?
Have you had a stroke, transient ischemic attacks (TIAs) or Raynaud syndrome?
Do you have high blood pressure?
Have you ever had to stop taking this or any other medication because of an allergy or any other problems?
Are you taking any other medications, including other 5-HT1 agonists or any other migraine medications containing ergotamine,
dihydroergotamine, or methysergide.
Are you taking any medication for depression (monoamine oxidase inhibitors or selective serotonin reuptake inhibitors [SSRIs])?
Have you had, or do you have, any disease of the liver or kidney?
Have you had, or do you have, epilepsy or seizures?
Is this headache different from your usual migraine attacks?
Remember, if you answered YES to any of the above questions, then discuss it with your doctor.
The Use of Sumatriptan Succinate During Pregnancy
Do not use sumatriptan succinate injection, tablets, or nasal spray if you are pregnant, think you might be pregnant, are trying to
become pregnant, or are not using adequate contraception, unless you have discussed this with your doctor.
How to Use Sumatriptan Succinate?
Injection: Before injecting sumatriptan succinate, check with your doctor on acceptable injection sites and see the instructions (on
or inside the carton) on discarding empty syringes and loading an autoinjector device.
Never reuse a syringe.
For adults, the usual dose is a single injection given just below the skin. It should be given as soon as the symptoms of your migraine
appear, but may be given at any time during an attack. A second injection may be given if your symptoms of migraine come back. If your
symptoms do not improve following the first injection, do not give a second injection for the same attack without first consulting with
your doctor. Do not have more than two injections in any 24 hours and allow at least 1 hour between each dose.
Tablets: For adults, the usual dose is a single tablet taken whole with fluids. A second tablet may be taken if your symptoms of migraine
come back or if you have a partial response to the initial dose, but not sooner than 2 hours following the first tablet. For a given
attack, if you have no response to the first tablet, do not take a second tablet without first consulting with your doctor. Do not take
more than a total of 200 mg of sumatriptan succinate tablets in any 24-hour period. The safety of treating an average of more than four
headaches in a 30-day period has not been established.
Nasal Spray: Before using sumatriptan nasal spray, see the enclosed instruction pamphlet. For adults, the usual dose is a single nasal
spray administered into one nostril. If your headache comes back, a second nasal spray may be administered anytime after 2 hours of
administering the first spray. For any attack where you have no response to the first nasal spray, do not take a second nasal spray
without first consulting with your doctor. Do not administer more than a total of 40 mg of sumatriptan nasal spray in any 24-hour
period. The effects of long-term repeated use of sumatriptan nasal spray on the surfaces of the nose and throat have not been specifically
studied. The safety of treating an average of more than four headaches in a 30-day period has not been established.
Side Effects to Watch For
Some patients experience pain or tightness in the chest or throat when using sumatriptan succinate injection, tablets, or nasal spray.
If this happens to you, then discuss it with your doctor before using any more sumatriptan succinate. If the chest pain is severe or
does not go away, call your doctor immediately.
If you have sudden and/or severe abdominal pain following sumatriptan succinate, call your doctor immediately.
Shortness of breath; wheeziness; heart throbbing; swelling of eyelids, face, or lips; or a skin rash, skin lumps, or hives happen
rarely. If it happens to you, then tell your doctor immediately. Do not take any more sumatriptan succinate injection, tablets, or
nasal spray unless your doctor tells you to do so.
Some people may have feelings of tingling, heat, flushing (redness of face lasting a short time), heaviness or pressure after
treatment with sumatriptan succinate injection, tablets, or nasal spray. A few people may feel drowsy, dizzy, tired or sick, (or
experience nasal irritation with the nasal spray only). Tell your doctor of these symptoms at your next visit.
If you should feel unwell in any other way or have symptoms that you do not understand, you should contact your doctor immediately.
NOTE for Injection: You may experience pain or redness at site of injection, but this usually lasts less than an hour.
What to do if an Overdose is Taken
If you have taken more medication than you have been told, contact either your doctor, hospital emergency department, or nearest
poison control center immediately.
Storing Your Medicine
Keep your medicine in a safe place where children cannot reach it. It may be harmful to children. Store your medication from heat
and light. Tablets and Nasal Spray: Do not store at temperatures above 30�C (86�F) or below 2�C (36�F).
If your medication has expired (the expiration date is printed on the treatment pack), throw it away as instructed.
If your doctor decides to stop treatment, do not keep any leftover medicine unless your doctor tells you to. Throw away your medicine
as instructed.
For Injection: Do not throw away your autoinjector. Keep your medication in the case provided and do not store at temperatures above
30�C (86�F).
Imitrex Organismos afectados
Humanos y otros mamíferos