Fazaclo ODT




Categoria
Fazaclo ODT Nombres de marca, Fazaclo ODT Analogos
Fazaclo ODT Marca los nombres de mezcla
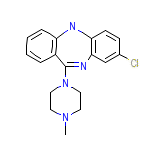
Fazaclo ODT Formula quimica
C18H19ClN4
Fazaclo ODT RX enlace
http://www.rxlist.com/cgi/generic3/clozapine.htm
Fazaclo ODT FDA hoja
Fazaclo ODT MSDS (hoja de seguridad de materiales)
Fazaclo ODT Sintesis de referencia
No hay información disponible
Fazaclo ODT Peso molecular
326.823 g/mol
Fazaclo ODT Punto de fusion
183-184 oC
Fazaclo ODT H2O Solubilidad
11.8 mg / L
Fazaclo ODT Estado
Solid
Fazaclo ODT LogP
2.502
Fazaclo ODT Formas de dosificacion
Tableta
Fazaclo ODT Indicacion
Para el manejo de los pacientes esquizofrénicos gravemente enfermos que no responden adecuadamente al tratamiento farmacológico estándar para la esquizofrenia
Fazaclo ODT Farmacologia
La clozapina es un agente psicotrópico que pertenece a la clase química de derivados del benzisoxazol y está indicado para el tratamiento de la esquizofrenia. La clozapina es un antagonista selectivo con monoaminérgicos alta afinidad por la serotonina tipo 2 (5HT2), la dopamina tipo 2 (D2), 1 y 2 adrenérgicos y los receptores histaminérgicos H1. Clozapina actúa como un antagonista de los receptores, pero con menor potencia. Antagonismo en otros receptores de dopamina y 5HT2 con afinidades receptoras similares puede explicar algunos de los otros efectos terapéuticos y secundarios de la clozapina. Clozapina antagonismo de receptores muscarínicos M1-5 receptores pueden explicar sus efectos anticolinérgicos. Clozapina antagonismo de los receptores H1 de histamina puede explicar la somnolencia observada con esta droga. Clozapina antagonismo de los receptores adrenérgicos a1 puede explicar la hipotensión ortostática observada con esta droga.
Fazaclo ODT Absorcion
Rápida y casi completa
Fazaclo ODT Toxicidad
No hay información disponible
Fazaclo ODT Informacion de Pacientes
PATIENT INFORMATION
� Patients who are to receive CLOZARIL should be warned about the significant risk of developing agranulocytosis. They should be informed that weekly blood tests are required for the first 6 months, if acceptable WBC counts (WBC � 3000/mm3, ANC � 1500/mm3) have been maintained during the first 6 months of continuous therapy, then WBC counts can be monitored every other week in order to monitor for the occurrence of agranulocytosis, and that CLOZARIL tablets will be made available only through a special program designed to ensure the required blood monitoring. Patients should be advised to report immediately the appearance of lethargy, weakness, fever, sore throat, malaise, mucous membrane ulceration or other possible signs of infection.
Particular attention should be paid to any flu-like complaints or other symptoms that might suggest infection.
� Patients should be informed of the significant risk of seizure during CLOZARIL treatment, and they should be advised to avoid driving and any other potentially hazardous activity while taking CLOZARIL.
� Patients should be advised of the risk of orthostatic hypotension, especially during the period of initial dose titration.
� Patients should be informed that if they stop taking CLOZARIL for more than 2 days, they should not restart their medication at the same dosage, but should contact their physician for dosing instructions.
� Patients should notify their physician if they are taking, or plan to take, any prescription or over-the-counter drugs or alcohol.
� Patients should notify their physician if they become pregnant or intend to become pregnant during therapy.
� Patients should not breast-feed an infant if they are taking CLOZARIL.
� Patients who are to receive CLOZARIL should be warned about the significant risk of developing agranulocytosis. They should be informed that weekly blood tests are required for the first 6 months, if acceptable WBC counts (WBC � 3000/mm3, ANC � 1500/mm3) have been maintained during the first 6 months of continuous therapy, then WBC counts can be monitored every other week in order to monitor for the occurrence of agranulocytosis, and that CLOZARIL tablets will be made available only through a special program designed to ensure the required blood monitoring. Patients should be advised to report immediately the appearance of lethargy, weakness, fever, sore throat, malaise, mucous membrane ulceration or other possible signs of infection.
Particular attention should be paid to any flu-like complaints or other symptoms that might suggest infection.
� Patients should be informed of the significant risk of seizure during CLOZARIL treatment, and they should be advised to avoid driving and any other potentially hazardous activity while taking CLOZARIL.
� Patients should be advised of the risk of orthostatic hypotension, especially during the period of initial dose titration.
� Patients should be informed that if they stop taking CLOZARIL for more than 2 days, they should not restart their medication at the same dosage, but should contact their physician for dosing instructions.
� Patients should notify their physician if they are taking, or plan to take, any prescription or over-the-counter drugs or alcohol.
� Patients should notify their physician if they become pregnant or intend to become pregnant during therapy.
� Patients should not breast-feed an infant if they are taking CLOZARIL.
Fazaclo ODT Organismos afectados
Humanos y otros mamíferos