Zyflo




Zyflo Brand names, Zyflo Analogs
Zyflo Brand Names Mixture
- No information avaliable
Zyflo Chemical_Formula
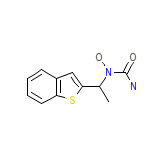
C11H12N2O2S
Zyflo RX_link
http://www.rxlist.com/cgi/generic/zileuton.htm
Zyflo fda sheet
Zyflo msds (material safety sheet)
Zyflo Synthesis Reference
No information avaliable
Zyflo Molecular Weight
236.291 g/mol
Zyflo Melting Point
144.2-145.2 oC
Zyflo H2O Solubility
Practically insoluble (0.5 mg/ml)
Zyflo State
Solid
Zyflo LogP
1.437
Zyflo Dosage Forms
Tablet (600mg)
Zyflo Indication
For the prophylaxis and chronic treatment of asthma in adults and children 12 years of age and older.
Zyflo Pharmacology
Zileuton is an asthma drug. It blocks leukotriene synthesis by inhibiting 5-lipoxygenase, an enzyme of the eicosanoid synthesis pathway. Sulfido-peptide leukotrienes (LTC4, LTD4, LTE4, also known as the slow-releasing substances of anaphylaxis) and LTB4, a chemoattractant for neutrophils and eosinophils, can be measured in a number of biological fluids including bronchoalveolar lavage fluid (BALF) from asthmatic patients. In humans, pretreatment with zileuton attenuated bronchoconstriction caused by cold air challenge in patients with asthma.
Zyflo Absorption
Rapidly and almost completely absorbed. The absolute bioavailability is unknown.
Zyflo side effects and Toxicity
The oral minimum lethal doses in mice and rats were 500-4000 and 300-1000 mg/kg in various preparations, respectively (providing greater than 3 and 9 times the systemic exposure [AUC] achieved at the maximum recommended human daily oral dose, respectively).
Zyflo Patient Information
Patients should be told that:
- ZYFLO is indicated for the chronic treatment of asthma and should be taken regularly as prescribed, even during symptom-free periods.
- ZYFLO is not a bronchodilator and should not be used to treat acute episodes of asthma.
- When taking ZYFLO, they should not decrease the dose or stop taking any other antiasthma medications unless instructed by a physician.
- While using ZYFLO, medical attention should be sought if short-acting bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for a 24-hour period are needed.
- The most serious side effect of ZYFLO is elevation of liver enzyme tests and that, while taking ZYFLO, they must return for liver enzyme test monitoring on a regular basis. If they experience signs and/or symptoms of liver dysfunction (e.g., right upper quadrant pain, nausea, fatigue, lethargy, pruritus, jaundice, or "flu-like" symptoms), they should contact their physician immediately.
- ZYFLO can interact with other drugs and that, while taking ZYFLO, they should consult their doctor before starting or stopping any prescription or non-prescription medicines.
A patient leaflet is included with the tablets.
Zyflo Organisms Affected
Humans and other mammals