Zeffix




Zeffix Brand names, Zeffix Analogs
Zeffix Brand Names Mixture
- Combivir (Lamivudine + Zidovudine)
- Kivexa (Abacavir (Abacavir Sulfate) + Lamivudine)
- Trizivir (Abacavir (Abacavir Sulfate) + Lamivudine + Zidovudine)
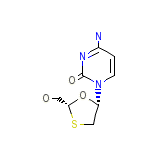
Zeffix Chemical_Formula
C8H11N3O3S
Zeffix RX_link
http://www.rxlist.com/cgi/generic3/lamivudine.htm
Zeffix fda sheet
Zeffix msds (material safety sheet)
Zeffix Synthesis Reference
No information avaliable
Zeffix Molecular Weight
229.257 g/mol
Zeffix Melting Point
160-162 oC
Zeffix H2O Solubility
70 mg/ml
Zeffix State
Solid
Zeffix LogP
No information avaliable
Zeffix Dosage Forms
Solution; Tablet
Zeffix Indication
For the treatment of HIV infection and chronic hepatitis B (HBV).
Zeffix Pharmacology
Lamivudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1) and hepatitis B (HBV). Lamivudine is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. The lack of a 3'-OH group in the incorporated nucleoside analogue prevents the formation of the 5' to 3' phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated.
Zeffix Absorption
Lamivudine was rapidly absorbed after oral administration in HIV-infected patients. Absolute bioavailability in adults is 86% ± 16% for the tablet and 87% ± 13% for the oral solution.
Zeffix side effects and Toxicity
No information avaliable
Zeffix Patient Information
Zeffix Organisms Affected
Human immunodeficiency virus and Hepatitis B virus