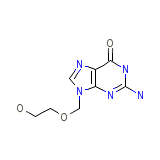
Vipral




Vipral Brand names, Vipral Analogs
Vipral Brand Names Mixture
- Zovirax Injection (Zovirax sodium)
Vipral Chemical_Formula
C8H11N5O3
Vipral RX_link
http://www.rxlist.com/cgi/generic/acyclo.htm
Vipral fda sheet
Vipral msds (material safety sheet)
Vipral Synthesis Reference
H. Matsumoto et al., Chem. Pharm. Bull. 35, 1153 (1988)
Vipral Molecular Weight
225.205 g/mol
Vipral Melting Point
256.5 - 257 oC
Vipral H2O Solubility
3.399 mg/L
Vipral State
Solid
Vipral LogP
-1.314
Vipral Dosage Forms
Intravenous infusion; Capsule; Tablet; Suspension; Topical cream; Topical ointment
Vipral Indication
For the treatment and management of herpes zoster (shingles), genital herpes, and chickenpox
Vipral Pharmacology
Acyclovir is a synthetic deoxyguanosine analog and it is the prototype antiviral agent that is activated by viral thymidine kinase. The selective activity of acyclovir is due to its affinity for the thymidine kinase enzyme encoded by HSV and VZV.
Vipral Absorption
Oral: bioavailability 10 to 20%
Vipral side effects and Toxicity
Acyclovir may cause nephrotoxicity (crystallization of acyclovir within renal tubules, elevation of serum creatinine, transient), and neurotoxicity (coma, hallucinations, lethargy, seizures, tremors). Nephrotoxicity and neurotoxicity usually resolve after cessation of acyclovir therapy. However, there is no well-defined relationship between acyclovir concentrations in the blood and these adverse effects.
Vipral Patient Information
Vipral Organisms Affected
Human herpes virus