Vesdil




Vesdil Brand names, Vesdil Analogs
Vesdil Brand Names Mixture
- No information avaliable
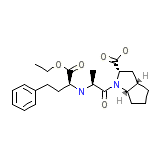
Vesdil Chemical_Formula
Vesdil RX_link
Vesdil fda sheet
Vesdil msds (material safety sheet)
Vesdil Synthesis Reference
Vesdil Molecular Weight
Vesdil Melting Point
Vesdil H2O Solubility
Vesdil State
Vesdil LogP
Vesdil Dosage Forms
Vesdil Indication
Vesdil Pharmacology
Vesdil Absorption
Vesdil side effects and Toxicity
Vesdil Patient Information
Pregnancy: Female patients of childbearing age should be told about the consequences of second- and third-trimester exposure to ACE inhibitors, and they should also be told that these consequences do not appear to have resulted from intrauterine ACE-inhibitor exposure that has been limited to the first trimester. These patients should be asked to report pregnancies to their physicians as soon as possible.
Angioedema: Angioedema, including laryngeal edema, can occur with treatment with ACE inhibitors, especially following the first dose. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, eyes, lips, or tongue, or difficulty in breathing) and to take no more drug until they have consulted with the prescribing physician.
Symptomatic Hypotension: Patients should be cautioned that lightheadedness can occur, especially during the first days of therapy, and it should be reported. Patients should be told that if syncope occurs, ramipril should be discontinued until the physician has been consulted.
All patients should be cautioned that inadequate fluid intake or excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Hyperkalemia: Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Neutropenia: Patients should be told to promptly report any indication of infection (e.g., sore throat, fever), which could be a sign of neutropenia.