Vanceril Double Strength




Vanceril Double Strength Brand names, Vanceril Double Strength Analogs
- Aerobec
- Aldecin
- Anceron
- Andion
- Beclacin
- Becloforte
- Beclomet
- Beclometasona [Inn-Spanish]
- Beclometasondipropionat Mikron
- Beclometasondipropionat Mikron.
- Beclometasone
- Beclometasonum [Inn-Latin]
- Beclorhinol
- Becloval
- Beclovent
- Becodisks
- Beconase
- Beconase AQ
- Beconasol
- Becotide
- Clenil-A
- Entyderma
- Inalone
- Korbutone
- Propaderm
- Qvar
- Qvar 80
- Rino-Clenil
- Sanasthmax
- Sanasthmyl
- Vancenase
- Vancenase AQ
- Vanceril
- Vanceril Double Strength
- Viarex
- Viarox
Vanceril Double Strength Brand Names Mixture
- none
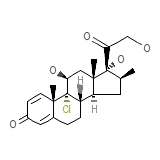
Vanceril Double Strength Chemical_Formula
C28H37ClO7
Vanceril Double Strength RX_link
http://www.rxlist.com/cgi/generic2/qvar.htm
Vanceril Double Strength fda sheet
Vanceril Double Strength msds (material safety sheet)
Vanceril Double Strength Synthesis Reference
U.S. Pat. 3,312,590 (1967)
Vanceril Double Strength Molecular Weight
521.042 g/mol
Vanceril Double Strength Melting Point
117-120oC
Vanceril Double Strength H2O Solubility
49.39 mg/L
Vanceril Double Strength State
Solid
Vanceril Double Strength LogP
3.795
Vanceril Double Strength Dosage Forms
Oral inhalation; Nasal inhalation; Nasal spray
Vanceril Double Strength Indication
For the maintenance treatment of asthma as prophylactic therapy in patients 5 years of age and older
Vanceril Double Strength Pharmacology
Beclomethasone, a synthetic halogenated glucocorticoid with antiinflammatory and vasoconstrictive effects, is used for treating steroid-dependent asthma, allergic or nonallergic rhinitis, or recurrent nasal polyps.
Vanceril Double Strength Absorption
Mean peak plasma concentration was 88pg/ml at 0.5 hour
Vanceril Double Strength side effects and Toxicity
The acute toxicity of beclomethasone dipropionate is low. The only harmful effect that follows inhalation of large amounts of the drug over a short period of time is suppression of hypothalamic-pituitary-adrenal (HPA) function. Chronic: The excessive use of beclomethasone dipropionate over a long period could lead to adrenal suppression.
Vanceril Double Strength Patient Information
Vanceril Double Strength Organisms Affected
Humans and other mammals