Valstar




Valstar Brand names, Valstar Analogs
Valstar Brand Names Mixture
- No information avaliable
Valstar Chemical_Formula
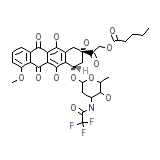
C34H36F3NO13
Valstar RX_link
http://www.rxlist.com/cgi/generic2/rubicin.htm
Valstar fda sheet
Valstar msds (material safety sheet)
Valstar Synthesis Reference
No information avaliable
Valstar Molecular Weight
723.644 g/mol
Valstar Melting Point
116-117oC
Valstar H2O Solubility
insoluble
Valstar State
Solid
Valstar LogP
3.332
Valstar Dosage Forms
Liquid
Valstar Indication
Bladder cancer
Valstar Pharmacology
When 800 mg Valstar was administered intravesically to patients with carcinoma in situ, Valstar penetrated into the bladder wall. The mean total anthracycline concentration measured in bladder tissue exceeded the levels causing 90% cytoxicity to human bladder cells cultured in vitro. During the two-hour dose-retention period, the metabolism of Valstar to its major metabolites N-trifluoroacetyladriamycin and N-trifluoroacetyladriamycinol was neglible. After retention, the drug was almost completely excreted by voiding the instillate. Mean percent recovery of Valstar, N-trifluoroacetyladriamycin, and total anthracyclines in 14 urine samples from six patients was 98.6%, 0.4%, and 99.0% of the total administered drug, respectively. During the two-hour dose-retention period, only nanogram quantities of Valstar were absorbed into the plasma.
Valstar Absorption
No information avaliable
Valstar side effects and Toxicity
No information avaliable
Valstar Patient Information
Valstar Organisms Affected
Humans and other mammals