V-Sul




V-Sul Brand names, V-Sul Analogs
- Accuzole
- Alphazole
- Amidoxal
- Astrazolo
- Azo Gantrisin
- Azosulfizin
- Bactesulf
- Barazae
- Chemouag
- Cosoxazole
- Dorsulfan
- Dorsulfan Warthausen
- Entusil
- Entusul
- G-Sox
- Ganda
- Gantrisin
- Gantrisine
- Gantrisona
- Gantrosan
- Isoxamin
- J-Sul
- Koro-Sulf
- Neazolin
- Neoxazol
- Norilgan-S
- Novazolo
- Novosaxazole
- Pancid
- Renosulfan
- Resoxol
- Roxosul
- Roxosul Tablets
- Roxoxol
- SI
- Saxosozine
- Sk-Soxazole
- Sodizole
- Sosol
- Soxamide
- Soxazole
- Soxisol
- Soxitabs
- Soxo
- Soxomide
- Stansin
- Sulbio
- Sulfadimethylisoxazole
- Sulfafurazol
- Sulfafurazole
- Sulfagan
- Sulfagen
- Sulfaisoxazole
- Sulfalar
- Sulfapolar
- Sulfasan
- Sulfasol
- Sulfasoxazole
- Sulfazin
- Sulfisin
- Sulfisonazole
- Sulfisoxasole
- Sulfisoxazol
- Sulfisoxazole Dialamine
- Sulfisoxazole Diolamine
- Sulfizin
- Sulfizol
- Sulfizole
- Sulfofurazole
- Sulfoxol
- Suloxsol
- Sulphadimethylisoxazole
- Sulphafuraz
- Sulphafurazol
- Sulphafurazole
- Sulphafurazolum
- Sulphaisoxazole
- Sulphisoxazol
- Sulphofurazole
- Sulsoxin
- Thiasin
- Tl-Azole
- Unisulf
- Urisoxin
- Uritrisin
- Urogan
- V-Sul
- Vagilia
V-Sul Brand Names Mixture
- Pediazole (Erythromycin (Erythromycin Ethylsuccinate) + Sulfisoxazole Acetyl)
- Renazone Plus Tab (Ammonium Chloride + Atropine Sulfate + Hyoscyamine Hydrobromide + Scopolamine Hydrobromide + Sulfisoxazole)
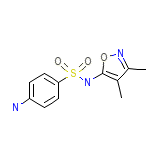
V-Sul Chemical_Formula
C11H13N3O3S
V-Sul RX_link
No information avaliable
V-Sul fda sheet
V-Sul msds (material safety sheet)
V-Sul Synthesis Reference
H. M. Wuest, M. Hoffer, U.S. pat. 2,430,094 (1947 to Hoffmann-La Roche)
V-Sul Molecular Weight
267.305 g/mol
V-Sul Melting Point
194 oC
V-Sul H2O Solubility
0.13 mg/ml (25 oC)
V-Sul State
Solid
V-Sul LogP
2.569
V-Sul Dosage Forms
Tablet; Oral suspension; Eye drops
V-Sul Indication
Treatment of severe, repeated, or long-lasting urinary tract infections, meningococcal meningitis, acute otitis media, trachoma, inclusion conjunctivitis, nocardiosis, chancroid, toxoplasmosis, malaria and other bacterial infections.
V-Sul Pharmacology
Sulfisoxazole is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
V-Sul Absorption
No information avaliable
V-Sul side effects and Toxicity
LD50=6800 mg/kg (Orally in mice)
V-Sul Patient Information
V-Sul Organisms Affected
Enteric bacteria and other eubacteria