Usaf ea-1




Usaf ea-1 Brand names, Usaf ea-1 Analogs
- Bifuron
- Corizium
- Coryzium
- Diafuron
- Enterotoxon
- Fiurox aerosol powder
- Furall
- Furaxon
- Furaxone
- Furazol
- Furazolidine
- Furazolidon
- Furazon
- Furidon
- Furovag
- Furox
- Furoxal
- Furoxane
- Furoxon
- Furoxone
- Furoxone Liquid
- Furoxone Swine Mix
- Furozolidine
- Giardil
- Giarlam
- Medaron
- Neftin
- Nicolen
- Nifulidone
- Nifuran
- Nifurazolidone
- Nitrofurazolidone
- Nitrofurazolidonum
- Nitrofuroxon
- Optazol
- Ortazol
- Puradin
- Roptazol
- Sclaventerol
- Tikofuran
- Topazone
- Trichofuron
- Tricofuron
- Tricoron
- Trifurox
- Usaf ea-1
- Viofuragyn
Usaf ea-1 Brand Names Mixture
- No information avaliable
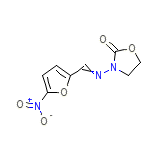
Usaf ea-1 Chemical_Formula
C8H7N3O5
Usaf ea-1 RX_link
http://www.rxlist.com/cgi/generic2/furazol.htm
Usaf ea-1 fda sheet
Usaf ea-1 msds (material safety sheet)
Usaf ea-1 Synthesis Reference
No information avaliable
Usaf ea-1 Molecular Weight
225.158 g/mol
Usaf ea-1 Melting Point
255 oC
Usaf ea-1 H2O Solubility
40 mg/L
Usaf ea-1 State
Solid
Usaf ea-1 LogP
0.691
Usaf ea-1 Dosage Forms
Tablets; Liquid
Usaf ea-1 Indication
For the specific and symptomatic treatment of bacterial or protozoal diarrhea and enteritis caused by susceptible organisms.
Usaf ea-1 Pharmacology
Furoxone has a broad antibacterial spectrum covering the majority of gastrointestinal tract pathogens including E. coli, staphylococci, Salmonella, Shigella, Proteus, Aerobacter aerogenes, Vibrio cholerae and Giardia lamblia. Its bactericidal activity is based upon its interference with DNA replication and protein production; this antimicrobial action minimizes the development of resistant organisms.
Usaf ea-1 Absorption
Radiolabeled drug studies indicate that furazolidone is well absorbed following oral administration
Usaf ea-1 side effects and Toxicity
Reactions to Furoxone have been reported including a fall in blood pressure, urticaria, fever, arthralgia, and a vesicular morbilliform rash. Other adverse effects can include a brown discoloration of the urine; hemolysis can occur in G6PDH-deficient patients. The drug has a monoamine oxidase (MAO) inhibitory effect and should never be given concurrently to individuals already taking MAO inhibitors.
Usaf ea-1 Patient Information
No information avaliable
Usaf ea-1 Organisms Affected
Microbes (bacteria, parasites)