Ultiva




Ultiva Brand names, Ultiva Analogs
Ultiva Brand Names Mixture
- No information avaliable
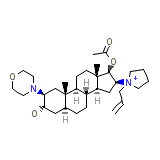
Ultiva Chemical_Formula
C32H53N2O4+
Ultiva RX_link
http://www.rxlist.com/cgi/generic2/rocuronium.htm
Ultiva fda sheet
Ultiva msds (material safety sheet)
Ultiva Synthesis Reference
No information avaliable
Ultiva Molecular Weight
529.774 g/mol
Ultiva Melting Point
No information avaliable
Ultiva H2O Solubility
Complete
Ultiva State
Solid
Ultiva LogP
No information avaliable
Ultiva Dosage Forms
Solution (for intravenous injection containing 10mg rocuronium bromide/mL solution)
Ultiva Indication
For inpatients and outpatients as an adjunct to general anesthesia to facilitate both rapid sequence and routine tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Ultiva Pharmacology
Rocuronium is a nondepolarizing neuromuscular blocking agent with a rapid to intermediate onset depending on dose and intermediate duration
Ultiva Absorption
No information avaliable
Ultiva side effects and Toxicity
No cases of significant accidental or intentional overdose have been reported. Overdosage with neuromuscular blocking agents may result in neuromuscular block beyond the time needed for surgery and anesthesia.
Ultiva Patient Information
Ultiva Organisms Affected
Humans and other mammals