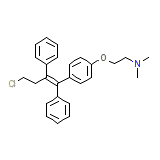
Toremifene




Toremifene Brand names, Toremifene Analogs
Toremifene Brand Names Mixture
- No information avaliable
Toremifene Chemical_Formula
C26H28ClNO
Toremifene RX_link
http://www.rxlist.com/cgi/generic2/toremifene.htm
Toremifene fda sheet
Toremifene msds (material safety sheet)
Toremifene Synthesis Reference
R. J. Toivola et al., Eur. pat. Appl. 95,875; U.S. pat. 4,696,949 (1983, 1087 both to Farmos)
Toremifene Molecular Weight
405.959 g/mol
Toremifene Melting Point
108-110 oC
Toremifene H2O Solubility
No information avaliable
Toremifene State
Solid
Toremifene LogP
6.725
Toremifene Dosage Forms
Tablet
Toremifene Indication
For the treatment of metastatic breast cancer in postmenopausal women with estrogen receptor-positive or receptor-unknown tumors
Toremifene Pharmacology
Toremifene is an antineoplastic hormonal agent primarily used in the treatment of advanced breast cancer. Toremifene is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effects may be related to its ability to compete with estrogen for binding sites in target tissues such as breast. Toremifene inhibits the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and causes the regression of already established DMBA-induced tumors. In this rat model, Toremifene appears to exert its antitumor effects by binding the estrogen receptors. In cytosols derived from human breast adenocarcinomas, Toremifene competes with estradiol for estrogen receptor protein.
Toremifene Absorption
Well absorbed
Toremifene side effects and Toxicity
No information avaliable
Toremifene Patient Information
Patient Information:
Vaginal bleeding has been reported in patients using Toremifene. Patients should be informed about
this and instructed to contact their physician if such bleeding occurs.
Patients with bone metastases should be informed about the typical signs and symptoms of hypercalcemia
and instructed to contact their physician for further assessment if such signs or symptoms occur.
Vaginal bleeding has been reported in patients using Toremifene. Patients should be informed about
this and instructed to contact their physician if such bleeding occurs.
Patients with bone metastases should be informed about the typical signs and symptoms of hypercalcemia
and instructed to contact their physician for further assessment if such signs or symptoms occur.
Toremifene Organisms Affected
Humans and other mammals