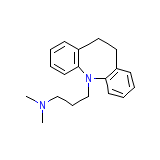
Tofranil, Base




Tofranil, Base Brand names, Tofranil, Base Analogs
- Antideprin
- Apo-Imipramine
- Berkomine
- Censtim
- Censtin
- DPID
- Declomipramine
- Dimipressin
- Dyna-Zina
- Dynaprin
- Estraldine
- Eupramin
- IM
- Imavate
- Imidobenzyle
- Imipramina
- Imipramine Hcl
- Imiprin
- Imizin
- Imizine
- Imizinum
- Impramine
- Impril
- Intalpram
- Iramil
- Irmin
- Janimine
- Melipramin
- Melipramine
- Nelipramin
- Norfranil
- Novopramine
- Pramine
- Prazepine
- Presamine
- Promiben
- Psychoforin
- Sk-Pramine
- Surmontil
- Surplix
- Timolet
- Tipramine
- Tofranil
- Tofranil, Base
- Tofranil-Pm
- Tofraniln A
- Trimipramine Maleate
Tofranil, Base Brand Names Mixture
- No information avaliable
Tofranil, Base Chemical_Formula
C19H24N2
Tofranil, Base RX_link
http://www.rxlist.com/cgi/generic/imip.htm
Tofranil, Base fda sheet
Tofranil, Base msds (material safety sheet)
Tofranil, Base Synthesis Reference
No information avaliable
Tofranil, Base Molecular Weight
280.407 g/mol
Tofranil, Base Melting Point
174.5 oC
Tofranil, Base H2O Solubility
18.2 mg/L
Tofranil, Base State
Solid
Tofranil, Base LogP
4.184
Tofranil, Base Dosage Forms
Tablet
Tofranil, Base Indication
For the relief of symptoms of depression and as temporary adjunctive therapy in reducing enuresis in children aged 6 years and older.
Tofranil, Base Pharmacology
Imipramine is a tricyclic antidepressant with general pharmacological properties similar to those of structurally related tricyclic antidepressant drugs such as amitriptyline and doxepin. A tertiary amine, imipramine inhibits the reuptake of serotonin more so than most secondary amine tricyclics, meaning that it blocks the reuptake of neurotransmitters serotonin and noradrenaline almost equally. It is also effective in migraine prophylaxis, but not in abortion of acute migraine attack.
Tofranil, Base Absorption
Rapidly and well absorbed after oral administration
Tofranil, Base side effects and Toxicity
Oral, rat LD50: 355 to 682 mg/kg. Toxic signs proceed progressively from depression, irregular respiration and ataxia to convulsions and death.
Tofranil, Base Patient Information
Tofranil, Base Organisms Affected
Humans and other mammals