Thalidomide




Thalidomide Brand names, Thalidomide Analogs
Thalidomide Brand Names Mixture
- No information avaliable
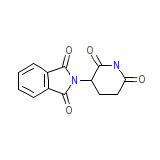
Thalidomide Chemical_Formula
C13H10N2O4
Thalidomide RX_link
http://www.rxlist.com/cgi/generic2/thalidom.htm
Thalidomide fda sheet
Thalidomide msds (material safety sheet)
Thalidomide Synthesis Reference
No information avaliable
Thalidomide Molecular Weight
258.23 g/mol
Thalidomide Melting Point
270 oC
Thalidomide H2O Solubility
545 mg/L
Thalidomide State
Solid
Thalidomide LogP
-0.146
Thalidomide Dosage Forms
Tablets for oral administration (50 mg); Capsules for oral administration (50 mg, 100 mg and 200 mg)
Thalidomide Indication
For the acute treatment of the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). Also for use as maintenance therapy for prevention and suppression of the cutaneous manifestations of ENL recurrence.
Thalidomide Pharmacology
Thalidomide is an immunomodulatory agent with a spectrum of activity that is not fully characterized. Thalidomide is racemic — it contains both left and right handed isomers in equal amounts: one enantiomer is effective against morning sickness, and the other is teratogenic. The enantiomers are converted to each other in vivo. That is, if a human is given D-thalidomide or L-thalidomide, both isomers can be found in the serum. Hence, administering only one enantiomer will not prevent the teratogenic effect in humans.
Thalidomide Absorption
The absolute bioavailability has not yet been characterized in human subjects due to its poor aqueous solubility. In studies of both healthy volunteers and subjects with Hansen’s disease, the mean time to peak plasma concentrations (Tmax) ranged from 2.9 to 5.7 hours indicating that thalidomide is slowly absorbed from the gastrointestinal tract.
Thalidomide side effects and Toxicity
The R-configuration and the S-configuration are more toxic individually than the racemic mixture. The LD50 could not be established in mice for racemic thalidomide, whereas LD50 values for the R and S configurations are reported to be 0.4 to 0.7 g/kg and 0.5 to 1.5 g/kg, respectively.
Thalidomide Patient Information
Thalidomide Organisms Affected
Humans and other mammals