Tetramisole




Tetramisole Brand names, Tetramisole Analogs
Tetramisole Brand Names Mixture
- No information avaliable
Tetramisole Chemical_Formula
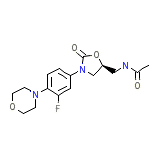
C16H20FN3O4
Tetramisole RX_link
http://www.rxlist.com/cgi/generic3/linezolid.htm
Tetramisole fda sheet
Tetramisole msds (material safety sheet)
Tetramisole Synthesis Reference
No information avaliable
Tetramisole Molecular Weight
337.346 g/mol
Tetramisole Melting Point
No information avaliable
Tetramisole H2O Solubility
3 mg/mL
Tetramisole State
Solid
Tetramisole LogP
0.232
Tetramisole Dosage Forms
Solution; Tablets
Tetramisole Indication
For the treatment of bacterial infections caused by susceptible strains of vancomycin resistant Enterococcus faecium, Staphylococcal aureus (methicillin resistant and susceptible strains), Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae.
Tetramisole Pharmacology
Linezolid is a synthetic antibacterial agent of a new class of antibiotics, the oxazolidinones, which has clinical utility in the treatment of infections caused by aerobic Gram-positive bacteria. The in vitro spectrum of activity of linezolid also includes certain Gram-negative bacteria and anaerobic bacteria. Susceptible organisms include methicillin- and vancomycin-resistant staphylococci, vancomycin-resistant enterococci, penicillin-resistant pneumococci and anaerobes. Oxazolidinones inhibit protein synthesis by binding at the P site at the ribosomal 50S subunit. Resistance to other protein synthesis inhibitors does not affect oxazolidinone activity, however rare development of oxazolidinone resistance cases, associated with 23S rRNA alterations during treatment have been reported. Linezolid inhibits bacterial protein synthesis through a mechanism of action different from that of other antibacterial agents; therefore, cross-resistance between linezolid and other classes of antibiotics is unlikely.
Tetramisole Absorption
Linezolid is rapidly and extensively absorbed after oral dosing. Maximum plasma concentrations are reached approximately 1 to 2 hours after dosing, and the absolute bioavailability is approximately 100%.
Tetramisole side effects and Toxicity
Clinical signs of acute toxicity lead to decreased activity, ataxia, vomiting and tremors.
Tetramisole Patient Information
Tetramisole Organisms Affected
Gram negative and gram positive bacteria