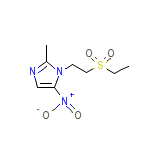
Tenofovir disoproxil fumarate




Tenofovir disoproxil fumarate Brand names, Tenofovir disoproxil fumarate Analogs
Tenofovir disoproxil fumarate Brand Names Mixture
- No information avaliable
Tenofovir disoproxil fumarate Chemical_Formula
C8H13N3O4S
Tenofovir disoproxil fumarate RX_link
No information avaliable
Tenofovir disoproxil fumarate fda sheet
Tenofovir disoproxil fumarate msds (material safety sheet)
Tenofovir disoproxil fumarate Synthesis Reference
No information avaliable
Tenofovir disoproxil fumarate Molecular Weight
247.273 g/mol
Tenofovir disoproxil fumarate Melting Point
127-128 oC
Tenofovir disoproxil fumarate H2O Solubility
1.99E+004 mg/L
Tenofovir disoproxil fumarate State
Solid
Tenofovir disoproxil fumarate LogP
0.119
Tenofovir disoproxil fumarate Dosage Forms
Pink film-coated oral tablets contain 500 mg or 250 mg of tinidazole.
Tenofovir disoproxil fumarate Indication
For the treatment of trichomoniasis caused by T. vaginalis in both female and male patients. Also for the treatment of giardiasis caused by G. duodenalis in both adults and pediatric patients older than three years of age and for the treatment of intestinal amebiasis and amebic liver abscess caused by E. histolytica in both adults and pediatric patients older than three years of age.
Tenofovir disoproxil fumarate Pharmacology
Tinidazole is a synthetic antiprotozoal agent. Tinidazole demonstrates activity both in vitro and in clinical infections against the following protozoa: Trichomonas vaginalis, Giardia duodenalis (also termed G. lamblia), and Entamoeba histolytica. Tinidazole does not appear to have activity against most strains of vaginal lactobacilli.
Tenofovir disoproxil fumarate Absorption
Rapidly and completely absorbed under fasting conditions. Administration with food results in a delay in Tmax of approximately 2 hours and a decline in Cmax of approximately 10% and an AUC of 901.6 ± 126.5 mcg hr/mL.
Tenofovir disoproxil fumarate side effects and Toxicity
There are no reported overdoses with tinidazole in humans. In acute studies with mice and rats, the LD 50 for mice was generally > 3,600 mg/kg for oral administration and was > 2,300 mg/kg for intraperitoneal administration. In rats, the LD 50 was > 2,000 mg/kg for both oral and intraperitoneal administration.
Tenofovir disoproxil fumarate Patient Information
Tenofovir disoproxil fumarate Organisms Affected
Trichomonas vaginalis, Giardia duodenalis (also termed G. lamblia), and Entamoeba histolytica