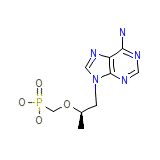
Tenofovir




Tenofovir Brand names, Tenofovir Analogs
Tenofovir Brand Names Mixture
- Didrocal (Calcium Carbonate + Etidronate Disodium)
Tenofovir Chemical_Formula
C9H14N5O4P
Tenofovir RX_link
http://www.rxlist.com/cgi/generic/viread.htm
Tenofovir fda sheet
Tenofovir msds (material safety sheet)
Tenofovir Synthesis Reference
No information avaliable
Tenofovir Molecular Weight
287.213 g/mol
Tenofovir Melting Point
276-280oC
Tenofovir H2O Solubility
13.4 mg/mL in distilled water at 25°C (disoproxil fumarate salt)
Tenofovir State
Solid
Tenofovir LogP
0.01
Tenofovir Dosage Forms
Tablets for oral administration (300 mg)
Tenofovir Indication
For use, in combination with other antiretroviral agents, for the treatment of HIV-1 infection.
Tenofovir Pharmacology
Tenofovir belongs to a class of antiretroviral drugs known as nucleotide analogue reverse transcriptase inhibitors (NtRTIs), which block reverse transcriptase, an enzyme crucial to viral production in HIV-infected people. Tenofovir is currently in late-stage clinical trials for the treatment of hepatitis B. Tenofovir disoproxil fumarate is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. Tenofovir requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Tenofovir Absorption
The oral bioavailability in fasted patients is approximately 25%. Administration of food (high fat meal containing 40 to 50% fat) increases the oral bioavailability, with an increase in the AUC of approximately 40%.
Tenofovir side effects and Toxicity
Limited clinical experience at doses higher than the therapeutic dose of tenofovir 300 mg is available. In Study 901 tenofovir disoproxil fumarate 600 mg was administered to 8 patients orally for 28 days. No severe adverse reactions were reported. The effects of higher doses are not known.
Tenofovir Patient Information
Tenofovir Organisms Affected
Human immunodeficiency virus