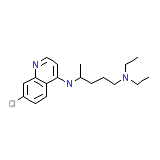
Tanakan




Tanakan Brand names, Tanakan Analogs
- 3377 RP opalate
- Amokin
- Aralen
- Aralen HCl
- Arechin
- Arthrochin
- Artrichin
- Avlochlor
- Avloclor
- Bemaco
- Bemaphate
- Bemasulph
- Benaquin
- Bipiquin
- Capquin
- Chemochin
- Chingamin
- Chloraquine
- Chlorochin
- Chlorochine
- Chloroquina
- Chloroquine (VAN)
- Chloroquine Phosphate
- Chloroquinium
- Chlorquin
- Cidanchin
- Clorochina
- Cocartrit
- Delagil
- Dichinalex
- Elestol
- Gontochin
- Heliopar
- Hydroxychloroquine Sulfate
- Imagon
- Iroquine
- Klorokin
- Lapaquin
- Malaquin
- Malaren
- Malarex
- Mesylith
- Neochin
- Nivachine
- Nivaquine
- Nivaquine B
- Nivaquine [as sulfate]
- Pfizerquine
- Plaquenil
- Quinachlor
- Quinagamin
- Quinagamine
- Quinercyl
- Quingamine
- Quinilon
- Quinoscan
- Resochen
- Resochin
- Resoquina
- Resoquine
- Reumachlor
- Reumaquin
- Roquine
- Sanoquin
- Silbesan
- Siragan
- Solprina
- Sopaquin
- Tanakan
- Tresochin
- Trochin
Tanakan Brand Names Mixture
- No information avaliable
Tanakan Chemical_Formula
C18H26ClN3
Tanakan RX_link
http://www.rxlist.com/cgi/generic2/hquine2.htm
Tanakan fda sheet
Tanakan msds (material safety sheet)
Tanakan Synthesis Reference
No information avaliable
Tanakan Molecular Weight
319.872 g/mol
Tanakan Melting Point
289 oC
Tanakan H2O Solubility
10.6 mg/L
Tanakan State
Solid
Tanakan LogP
4.474
Tanakan Dosage Forms
Tablet
Tanakan Indication
For the suppressive treatment and for acute attacks of malaria due to P. vivax, P.malariae, P. ovale, and susceptible strains of P. falciparum, Second-line agent in treatment of Rheumatoid Arthritis
Tanakan Pharmacology
Chloroquine is the prototype anti malarial drug, most widely used to treat all types of malaria except for disease caused by chloroquine resistant Plasmodium falciparum. It is highly effective against erythrocytic forms of Plasmodium vivax, Plasmodium ovale and Plasmodium malariae, sensitive strains of Plasmodium falciparum and gametocytes of Plasmodium vivax. Being alkaline, the drug reaches high concentration within the food vacuoles of the parasite and raises its pH. It is found to induce rapid clumping of the pigment. Chloroquine inhibits the parasitic enzyme heme polymerase that converts the toxic heme into non-toxic hemazoin, thereby resulting in the accumulation of toxic heme within the parasite. It may also interfere with the biosynthesis of nucleic acids.
Tanakan Absorption
Completely absorbed from gastrointestinal tract
Tanakan side effects and Toxicity
No information avaliable
Tanakan Patient Information
PATIENT INFORMATION
Complete blood cell counts should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered. The drug should be administered with caution to patients having G-6-PD (glucose-6 phosphate dehydrogenase) deficiency.
In patients with preexisting auditory damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed.
Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Patients with history of epilepsy should be advised about the risk of chloroquine provoking seizures.
Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother.
Irreversible retinal damage has been observed in some patients who had received long-term or high-dosage 4-aminoquinoline therapy. Retinopathy has been reported to be dose related.
Follow Rxlist link and drugs.com link for detailed patient information.
Complete blood cell counts should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered. The drug should be administered with caution to patients having G-6-PD (glucose-6 phosphate dehydrogenase) deficiency.
In patients with preexisting auditory damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed.
Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Patients with history of epilepsy should be advised about the risk of chloroquine provoking seizures.
Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother.
Irreversible retinal damage has been observed in some patients who had received long-term or high-dosage 4-aminoquinoline therapy. Retinopathy has been reported to be dose related.
Follow Rxlist link and drugs.com link for detailed patient information.
Tanakan Organisms Affected
Plasmodium