Sporicef




Sporicef Brand names, Sporicef Analogs
- Alcephin
- Alexin
- Alsporin
- Biocef
- CEX
- Carnosporin
- Cefa-iskia
- Cefablan
- Cefadal
- Cefadin
- Cefadina
- Cefaleksin
- Cefalessina [DCIT]
- Cefalexin
- Cefalexin (JP14)
- Cefalexin Sodium
- Cefalexina [INN-Spanish]
- Cefalexine [INN-French]
- Cefalexinum [INN-Latin]
- Cefalin
- Cefaloto
- Cefaseptin
- Cefax
- Ceforal
- Cefovit
- Celexin
- Cepastar
- Cepexin
- Cephacillin
- Cephalexin (USP)
- Cephalexin (free base)
- Cephalexin 1-hydrate
- Cephalexin hydrate
- Cephalexin monohydrate
- Cephalexine
- Cephalexinum
- Cephanasten
- Cephaxin
- Cephin
- Cepol
- Ceporex
- Ceporex Forte
- Ceporexin
- Ceporexin-E
- Ceporexine
- Check
- Cophalexin
- Durantel
- Durantel DS
- Ed A-Ceph
- Erocetin
- Factagard
- Felexin
- Fexin
- Ibilex
- Ibrexin
- Inphalex
- Kefalospes
- Keflet
- Keflex
- Kefolan
- Keforal
- Keftab
- Kekrinal
- Kidolex
- L-Keflex
- Lafarine
- Larixin
- Lenocef
- Lexibiotico
- Lonflex
- Lopilexin
- Madlexin
- Mamalexin
- Mamlexin
- Medoxine
- Neokef
- Neolexina
- Novolexin
- Nufex
- Oracef
- Oriphex
- Oroxin
- Ortisporina
- Ospexin
- Palitrex
- Panixine Disperdose
- Pectril
- Pyassan
- Roceph
- Roceph Distab
- Sanaxin
- Sartosona
- Sencephalin
- Sepexin
- Servispor
- Sialexin
- Sinthecillin
- Sporicef
- Sporidex
- Syncl
- Syncle
- Synecl
- Tepaxin
- Tokiolexin
- Uphalexin
- Voxxim
- Winlex
- Zozarine
Sporicef Brand Names Mixture
- No information avaliable
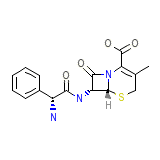
Sporicef Chemical_Formula
C16H17N3O4S
Sporicef RX_link
http://www.rxlist.com/cgi/generic/cephalex.htm
Sporicef fda sheet
Sporicef msds (material safety sheet)
Sporicef Synthesis Reference
R. B. Morin, B. G. Jackson, U.S. Pat. 3,275,626,861 (1966,1970)
Sporicef Molecular Weight
347.39 g/mol
Sporicef Melting Point
326.8oC
Sporicef H2O Solubility
1789 mg/L
Sporicef State
Solid
Sporicef LogP
0.019
Sporicef Dosage Forms
Capsules (containing cephalexin monohydrate equivalent to 250 mg or 500 mg cephalexin); Tablet (containing cephalexin monohydrate equivalent to 250 mg or 500 mg of cephalexin); Suspension
Sporicef Indication
For the treatment of respiratory tract infections caused by Streptococcus pneumoniae and Streptococcus pyogenes; otitis media due to Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pyogenes, and Moraxella catarrhalis; skin and skin structure infections caused by Staphylococcus aureus and/or Streptococcus pyogenes; bone infections caused by Staphylococcus aureus and/or Proteus mirabilis; genitourinary tract infections, including acute prostatitis, caused by Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae.
Sporicef Pharmacology
Cephalexin (also called Cefalexin) is a first generation cephalosporin antibiotic. It is one of the most widely prescribed antibiotics, often used for the treatment of superficial infections that result as complications of minor wounds or lacerations. It is effective against most gram-positive bacteria.
Sporicef Absorption
Well absorbed from the gastrointestinal tract
Sporicef side effects and Toxicity
Symptoms of overdose include blood in the urine, diarrhea, nausea, upper abdominal pain, and vomiting. The oral median lethal dose of cephalexin in rats is >5000 mg/kg.
Sporicef Patient Information
Patients should be counseled that antibacterial drugs including Keflex should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Keflex is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Keflex or other antibacterial drugs in the future.
Sporicef Organisms Affected
Enteric bacteria and other eubacteria