Sofro




Sofro Brand names, Sofro Analogs
- Aarane
- Acide Cromoglicique [Inn-French]
- Acido Cromoglicico [Inn-Spanish]
- Acidum Cromoglicicum [Inn-Latin]
- Alercom
- Alerion
- Allergocrom
- Apo-Cromolyn
- Children's Nasalcrom
- Colimune
- Crolom
- Cromoglicic Acid
- Cromoglycic Acid
- Cromolyn Nasal Solution
- Cromolyn Sodium
- Cromoptic
- Cromovet
- Diethyl Cromoglycicate
- Fivent
- Gastrocrom
- Gastrofrenal
- Gen-Cromoglycate
- Inostral
- Intal
- Intal Inhaler
- Intal Syncroner
- Introl
- Irtan
- Lomudal
- Lomupren
- Lomusol
- Lomuspray
- Nalcrom
- Nalcron
- Nasalcrom
- Nasmil
- Novo-Cromolyn
- Opticrom
- Opticron
- Pms-Sodium Cromoglycate
- Rynacrom
- Sodium Cromoglycate
- Sofro
- Vistacrom
- Vividrin
Sofro Brand Names Mixture
- No information avaliable
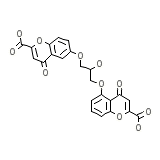
Sofro Chemical_Formula
Sofro RX_link
Sofro fda sheet
Sofro msds (material safety sheet)
Sofro Synthesis Reference
Sofro Molecular Weight
Sofro Melting Point
Sofro H2O Solubility
Sofro State
Sofro LogP
Sofro Dosage Forms
Sofro Indication
Sofro Pharmacology
Sofro Absorption
Sofro side effects and Toxicity
Sofro Patient Information
INTAL® INHALER (cromolyn sodium inhalation aerosol)
- Metered Dose Inhaler
- For Oral Inhalation Only
- Make sure the canister is properly inserted into the Inhaler unit. Take the cover off the mouthpiece. Shake the Inhaler gently. If the mouthpiece cover is not present, the Inhaler should be inspected for the presence of foreign objects.
- Hold Inhaler and breathe out slowly and fully, expelling as much air as possible. Do not breathe into the Inhaler - it could clog the Inhaler valve.
- Avoid spraying in eyes.
- Place the mouthpiece into your mouth, close your lips around it, and tilt your head back. Keep your tongue below the opening of the Inhaler.
- While breathing in deeply and slowly through the mouth, fully depress the top of the metal canister with your index finger.
- Remove the Inhaler from your mouth. Hold your breath for several seconds, then breathe out slowly. This step is very important. It allows the Intal to spread throughout your lungs. Repeat steps 2-5, then replace the mouthpiece cover.
FOR BEST RESULTS:
- Before using the Inhaler for the first time, or if it has not been used for a while, it's a good idea to test it. Just give the canister one press.
- It is essential that the canister be pressed at exactly the same time as you breathe in, so it's worth some time practicing this.
- The dose delivered from the Inhaler can be seen as a fine white mist. If any of this can be seen escaping from your mouth or nose, then you are not using the Inhaler correctly.
- To keep your Inhaler in good working order, do not exhale into mouthpiece.
- Keep the cap on the Inhaler while not in use so that dirt can't get into it. You can clean the Inhaler by removing the metal canister and rinsing the plastic mouthpiece in warm water.
- The correct amount of medication in each inhalation cannot be assured after 112 actuations from the 8.1 gram canister or 200 actuations from the 14.2 gram canister even though the canister may not feel completely empty. You should keep track of the number of actuations used from each canister of Intal Inhaler and discard the canister after 112 actuations from the 8.1 gram canister or 200 actuations from the 14.2 gram canister. Before you reach the specified number of actuations, you should consult your physician to determine whether a refill is needed. Just as you should not take extra doses without consulting your physician, you also should not stop using Intal Inhaler without consulting your physician.
- For optimal results, the canister should be at room temperature before use.
HOW TO CHECK CONTENTS OF YOUR CANISTER
Shaking the canister will NOT give you a good estimate of how much medication is left. We have included a convenient check-off chart to assist you in keeping track of medication inhalations used. This will help assure that you receive the labeled number of inhalations present.
- Each 8.1 gram Inhaler delivers 112 metered inhalations.
- Each 14.2 gram Inhaler delivers 200 metered inhalations.
Intal® Inhaler Check-Off Chart
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retain with medication or affix to convenient location.
Starting with inhalation #1, check off one number for each inhalation used.
DISCARD MEDICATION AFTER THE LABELED NUMBER OF INHALATIONS HAVE BEEN USED
NEVER IMMERSE THE METAL CANISTER IN WATER
IMPORTANT: Remember - a little time spent taking Intal correctly and regularly can save you from countless attacks of asthma and the upheaval they cause. It must be used every day as directed by your doctor. Do not stop the treatment or even reduce the dose without consulting your doctor. The Intal Inhaler canister and accompanying mouthpiece are designed to be used together. The Intal Inhaler canister should not be used with other mouthpieces and the supplied mouthpiece should not be used with other products' canisters.
DOSAGE: For management of bronchial asthma in adults and children 5 years of age and older, the usual
starting dosage is two metered inhalations four times a day at regular intervals. When asthma symptoms are well
controlled, your doctor may reduce the dose to three times a day, and sometimes two times a day.
For prevention of acute bronchospasm which follows exercise, exposure to cold, dry air, or environmental agents, the
usual dosage is two metered inhalations shortly before exposure to the offending factor. Use as directed by your
physician.
CLEANING: Twice a week, remove the metal canister from the plastic mouthpiece. Wash the mouthpiece in warm water and dry thoroughly before replacing the metal canister. Never immerse the metal canister in water.
STORAGE: Store between 15 to 30° C(59 to 86° F). Contents under pressure. Do not puncture, incinerate, or place near sources of heat. Exposure to temperatures above 120° F may cause bursting. Keep out of the reach of children. Avoid spraying in eyes.
Note: The indented statement below is required by the Federal government's Clean Air Act for all products contaning or manufactured with chlorofluorocarbons (CFCs).
This product contains CFC-12 (dichlorodifluoromethane) and CFC-114 (dichlorotetrafluoroethane), substances which harm the environment by destroying ozone in the upper atmosphere.Your physician has determined that this product is likely to help your personal health.
USE THIS PRODUCT AS DIRECTED, UNLESS INSTRUCTED TO DO OTHERWISE BY YOUR PHYSICIAN. If you have any questions about alternatives, consult with your physician.
Distributed by:
King Pharmaceuticalsô, Inc.
Bristol, TN 37620
Manufactured by:
Health Care Specialties Division
3M Health Care Limited
Loughborough, England LE11 1EP
©2002, 2003 Aventis Pharmaceuticals Inc.