Schebitran




Schebitran Brand names, Schebitran Analogs
- Achletin
- Anatran
- Anistadin
- Aponorin
- Carvacron
- Chlopolidine
- Cretonin
- Diu-Hydrin
- Diurazida
- Diurese
- Diuroral
- Esmarin
- Eurinol
- Fluitran
- Flurese
- Flutra
- Gangesol
- Hydrotrichlorothiazide
- Intromene
- Isestran
- Kubacron
- Metahydrin
- Metatensin
- Metatensin #2
- Metatensin #4
- Nakva
- Naqua
- Naquasone
- Naquival
- Salurin
- Schebitran
- Tachionin
- Tolcasone
- Trichlorex
- Trichlormas
- Trichlormetazid
- Trichlormethiazid
- Trichlormethiazide W/ Reserpine
- Trichloromethiadiazide
- Trichloromethiazide
- Triclordiuride
- Triclormetiazide
- Triflumen
Schebitran Brand Names Mixture
- No information avaliable
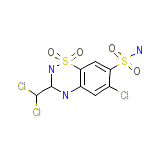
Schebitran Chemical_Formula
C8H8Cl3N3O4S2
Schebitran RX_link
No information avaliable
Schebitran fda sheet
Schebitran msds (material safety sheet)
Schebitran Synthesis Reference
No information avaliable
Schebitran Molecular Weight
380.657 g/mol
Schebitran Melting Point
No information avaliable
Schebitran H2O Solubility
No information avaliable
Schebitran State
Solid
Schebitran LogP
1.023
Schebitran Dosage Forms
No information avaliable
Schebitran Indication
Used in the treatment of oedema (including that associated with heart failure) and hypertension.
Schebitran Pharmacology
Trichloromethiazide is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. Trichloromethiazide has also been found useful in edema due to various forms of renal dysfunction such as nephrotic syndrome, acute glomer-ulonephritis, and chronic renal failure. Trichloromethiazide is also indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension. Like other thiazides, Trichloromethiazide promotes water loss from the body (diuretics). They inhibit Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.
Schebitran Absorption
No information avaliable
Schebitran side effects and Toxicity
Oral Rat LD50 = 5600 mg/kg, oral Mouse LD50 = 2600 mg/kg
Schebitran Patient Information
No information avaliable
Schebitran Organisms Affected
Humans and other mammals