SCM




SCM Brand names, SCM Analogs
SCM Brand Names Mixture
- No information avaliable
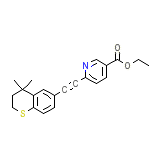
SCM Chemical_Formula
C21H21NO2S
SCM RX_link
http://www.rxlist.com/cgi/generic3/avage.htm
SCM fda sheet
SCM msds (material safety sheet)
SCM Synthesis Reference
No information avaliable
SCM Molecular Weight
351.463 g/mol
SCM Melting Point
No information avaliable
SCM H2O Solubility
Soluble
SCM State
Solid
SCM LogP
5.96
SCM Dosage Forms
Cream or gel (0.05% or 0.1%)
SCM Indication
Used to treat psoriasis, acne and sun damaged skin (photodamage).
SCM Pharmacology
Tazarotene is a prodrug and a member of the acetylenic class of retinoids. Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. When treating acne tazarotene may be taken in conjunction with an oral antibiotic. Tazarotene has been shown in peer-reviewed double blinded studies to reduce: mottling and hyperpigmentation, sallowness, fine wrinkling and coarse wrinkling in sun damaged skin. Histological studies have shown that long term (greater than 1 year) use of Tazarotene is associated with a significant reduction in atypical melanocytes and keratocytes - cells considered to be precursors of skin cancer. Some studies have shown long term use of Tazarotene to be associated with increased collagen production and better organization of skin collagen bundles.
SCM Absorption
Minimal systemic absorption of tazarotene occurs due to its rapid metabolism in the skin to the active metabolite, tazarotenic acid, which can be systemically absorbed and further metabolized. Gender had no influence on the systemic bioavailability of tazarotenic acid.
SCM side effects and Toxicity
Excessive topical use may lead to marked redness, peeling, or discomfort. Oral ingestion of the drug may affect liver function causing hypertriglyceridemia. Other symptoms may include conjunctival irritation, hair loss, headache, edema, fatigue, dermatitis, nausea, and visual disturbances. Oral administration of this material to rats and rabbits at doses of 0.20 mg/kg/day (rabbits) and 0.25 mg/kg/day (rats) resulted in developmental toxicity. A no effect level of 0.05 mg/kg/day was established. Similar teratogenic effects have been reported for other retinoid compounds.
SCM Patient Information
SCM Organisms Affected
Humans and other mammals