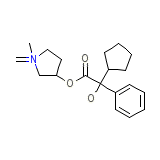
Robinul




Robinul Brand names, Robinul Analogs
Robinul Brand Names Mixture
- No information avaliable
Robinul Chemical_Formula
C19H28NO3+
Robinul RX_link
http://www.rxlist.com/cgi/generic3/glycopyrrolate.htm
Robinul fda sheet
Robinul msds (material safety sheet)
Robinul Synthesis Reference
No information avaliable
Robinul Molecular Weight
318.431 g/mol
Robinul Melting Point
192.5 oC
Robinul H2O Solubility
No information avaliable
Robinul State
Solid
Robinul LogP
-0.99
Robinul Dosage Forms
Liquid; Tablet
Robinul Indication
For use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions, to reduce the volume and free acidity of gastric secretions and to block cardiac vagal inhibitory reflexes during induction of anesthesia and intubation.
Robinul Pharmacology
Glycopyrrolate decreases acid secretion in the stomach. Hence it can be used for treating ulcers in the stomach and small intestine, in combination with other medications. In anesthesia, glycopyrrolate injection serves as a preoperative antimuscarinic operation that reduces salivary, tracheobronchial, and pharyngeal secretions, as well as decreases the acidity of gastric secretions blocks cardiac vagal inhibitory reflexes during intubation
Robinul Absorption
Rapidly absorbed (1-2 minutes) after intravenous injection
Robinul side effects and Toxicity
Side effects include dry mouth, difficult urinating, heachaches, diarrhea and constipation. The medication also induces drowsiness or blurred vision. LD50=709 mg/kg (rat, oral).
Robinul Patient Information
No information avaliable
Robinul Organisms Affected
Humans and other mammals