Rikavarin-S




Rikavarin-S Brand names, Rikavarin-S Analogs
Rikavarin-S Brand Names Mixture
- No information avaliable
Rikavarin-S Chemical_Formula
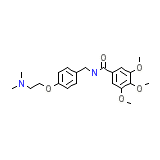
C21H28N2O5
Rikavarin-S RX_link
http://www.rxlist.com/cgi/generic3/trimethbenz.htm
Rikavarin-S fda sheet
Rikavarin-S msds (material safety sheet)
Rikavarin-S Synthesis Reference
No information avaliable
Rikavarin-S Molecular Weight
388.458 g/mol
Rikavarin-S Melting Point
188.7 oC
Rikavarin-S H2O Solubility
40 mg/L
Rikavarin-S State
Solid
Rikavarin-S LogP
2.784
Rikavarin-S Dosage Forms
Capsules (300 mg) for oral administration; Suppositories (200 mg); Injectable (100 mg, 200 mg)
Rikavarin-S Indication
For the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis.
Rikavarin-S Pharmacology
Trimethobenzamide is a novel antiemetic which prevents nausea and vomiting in humans. Its actions are unclear but most likely involves the chemoreceptor trigger zone (CTZ). In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate.
Rikavarin-S Absorption
The relative bioavailability of the capsule formulation compared to the solution is 100%.
Rikavarin-S side effects and Toxicity
Oral LD50 in mice is 1600 mg/kg.
Rikavarin-S Patient Information
No information avaliable
Rikavarin-S Organisms Affected
Humans and other mammals