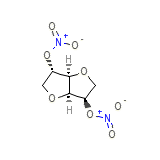
Resoidan




Resoidan Brand names, Resoidan Analogs
- Cardio 10
- Cardis
- Carvanil
- Carvasin
- Cedocard
- Claodical
- Cornilat
- Coronex
- Corosorbide
- Dianhydrosorbitol 2,5-dinitrate
- Difutrat
- Dilatrate
- Dilatrate-SR
- Dinitrosorbide
- Emoper
- Flindix
- Harrical
- IBD 20
- ISD
- ISDN
- Ismotic
- Iso-Bid
- Isochron
- Isoket
- Isorbid;
- Isordil
- Isordil Tembids
- Isosorbide dinitrate(USAN)
- Isostat
- Isotrate
- Korodil
- Lomilan
- Maycor
- Myorexon
- Nitrosorbid
- Nitrosorbide
- Nitrosorbon
- Nosim
- Resoidan
- Rifloc Retard
- Rigedal
- Sorbangil
- Sorbide T.D.
- Sorbide nitrate
- Sorbide, dinitrate
- Sorbidilat
- Sorbidnitrate
- Sorbislo
- Sorbitrate
- Sorbonit
- Sorquad
- Tinidil
- Titradose
- Vascardin
- Vasodilat
- Vasorbate
- Xanyl
Resoidan Brand Names Mixture
- No information avaliable
Resoidan Chemical_Formula
C6H8N2O8
Resoidan RX_link
http://www.rxlist.com/cgi/generic2/isodinit.htm
Resoidan fda sheet
Resoidan msds (material safety sheet)
Resoidan Synthesis Reference
Goldberg, Acta physiol. Scand.15, 173(1948)
Resoidan Molecular Weight
236.136 g/mol
Resoidan Melting Point
70 oC
Resoidan H2O Solubility
1.089 mg/mL
Resoidan State
Solid
Resoidan LogP
-0.53
Resoidan Dosage Forms
Oral tablet
Resoidan Indication
For the prevention of angina pectoris due to coronary artery disease.
Resoidan Pharmacology
Isosorbide Dinitrate is a moderate to long acting oral organic nitrate used for the relief and prophylactic management of angina pectoris. It relaxes the vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end- diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure.
Resoidan Absorption
Absorption of isosorbide dinitrate after oral dosing is nearly complete, but bioavailability is highly variable (10% to 90%), with extensive first-pass metabolism in the liver. The average bioavailability of isosorbide dinitrate is about 25%.
Resoidan side effects and Toxicity
Symptoms of overdose include reduced cardiac output and hypotension.
Resoidan Patient Information
PATIENT INFORMATION
Patients should be told that the anti-anginal efficacy of isosorbide dinitrate is strongly related
to its dosing regimen, so the prescribed schedule of dosing should be followed carefully. In
particular, daily headaches sometimes accompany treatment with isosorbide dinitrate. In patients
who get these headaches, the headaches are a marker of the activity of the drug. Patients should
resist the temptation to avoid headaches by altering the schedule of their treatment with isosorbide
dinitrate, since loss of headache may be associated with simultaneous loss of anti-anginal efficacy.
Aspirin and/or acetaminophen, on the other hand, often successfully relieve isosorbide dinitrate-
induced headaches with no deleterious effect an isosorbide dinitrate's anti-anginal efficacy.
Treatment with isosorbide dinitrate may be associated with lightheadedness on standing, especially
just after rising from a recumbent or seated position. This effect may be more frequent in patients
who have also consumed alcohol
Patients should be told that the anti-anginal efficacy of isosorbide dinitrate is strongly related
to its dosing regimen, so the prescribed schedule of dosing should be followed carefully. In
particular, daily headaches sometimes accompany treatment with isosorbide dinitrate. In patients
who get these headaches, the headaches are a marker of the activity of the drug. Patients should
resist the temptation to avoid headaches by altering the schedule of their treatment with isosorbide
dinitrate, since loss of headache may be associated with simultaneous loss of anti-anginal efficacy.
Aspirin and/or acetaminophen, on the other hand, often successfully relieve isosorbide dinitrate-
induced headaches with no deleterious effect an isosorbide dinitrate's anti-anginal efficacy.
Treatment with isosorbide dinitrate may be associated with lightheadedness on standing, especially
just after rising from a recumbent or seated position. This effect may be more frequent in patients
who have also consumed alcohol
Resoidan Organisms Affected
Humans and other mammals