Rebeprazole sodium




Rebeprazole sodium Brand names, Rebeprazole sodium Analogs
Rebeprazole sodium Brand Names Mixture
- No information avaliable
Rebeprazole sodium Chemical_Formula
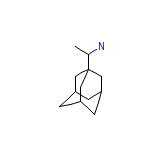
C12H21N
Rebeprazole sodium RX_link
http://www.rxlist.com/cgi/generic/rimantadine.htm
Rebeprazole sodium fda sheet
Rebeprazole sodium msds (material safety sheet)
Rebeprazole sodium Synthesis Reference
No information avaliable
Rebeprazole sodium Molecular Weight
179.302 g/mol
Rebeprazole sodium Melting Point
>300oC
Rebeprazole sodium H2O Solubility
Hydrochloride salt freely soluble (50 mg/ml at 20°C)
Rebeprazole sodium State
Solid
Rebeprazole sodium LogP
2.374
Rebeprazole sodium Dosage Forms
Available as 100 mg film-coated tablets and as syrup for oral administration.
Rebeprazole sodium Indication
For the prophylaxis and treatment of illness caused by various strains of influenza A virus in adults.
Rebeprazole sodium Pharmacology
Rimantadine, a cyclic amine, is a synthetic antiviral drug and a derivate of adamantane, like a similar drug amantadine. Rimantadine is inhibitory to the in vitro replication of influenza A virus isolates from each of the three antigenic subtypes (H1N1, H2H2 and H3N2) that have been isolated from man. Rimantadine has little or no activity against influenza B virus. Rimantadine does not appear to interfere with the immunogenicity of inactivated influenza A vaccine.
Rebeprazole sodium Absorption
Well absorbed, with the tablet and syrup formulations being equally absorbed after oral administration.
Rebeprazole sodium side effects and Toxicity
Oral LD50 in rats is 640 mg/kg. Overdoses of a related rug, amantadine, have been reported with adverse reactions consisting of agitation, hallucinations, cardiac arrhythmia and death.
Rebeprazole sodium Patient Information
Rebeprazole sodium Organisms Affected
Human Influenza A Virus