ReVia




ReVia Brand names, ReVia Analogs
ReVia Brand Names Mixture
- No information avaliable
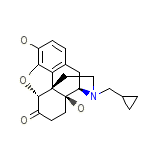
ReVia Chemical_Formula
ReVia RX_link
ReVia fda sheet
ReVia msds (material safety sheet)
ReVia Synthesis Reference
ReVia Molecular Weight
ReVia Melting Point
ReVia H2O Solubility
ReVia State
ReVia LogP
ReVia Dosage Forms
ReVia Indication
ReVia Pharmacology
ReVia Absorption
ReVia side effects and Toxicity
ReVia Patient Information
It is recommended that the prescribing physician relate the following information to patients being treated with REVIA:
You have been prescribed REVIA as part of the comprehensive treatment for your alcoholism or drug dependence. You should carry identification to alert medical personnel to the fact that you are taking REVIA. A REVIA medication card may be obtained from your physician and can be used for this purpose. Carrying the identification card should help to ensure that you can obtain adequate treatment in an emergency. If you require medical treatment, be sure to tell the treating physician that you are receiving REVIA therapy.
You should take REVIA as directed by your physician. If you attempt to self-administer heroin or any other opiate drug, in small doses while on REVIA, you will not perceive any effect. Most important, however, if you attempt to self-administer large doses of heroin or any other opioid while on REVIA, you may die or sustain serious injury, including coma.
REVIA is well-tolerated in the recommended doses, but may cause liver injury when taken in excess or in people who develop liver disease from other causes. If you develop abdominal pain lasting more than a few days, white bowel movements, dark urine, or yellowing of your eyes, you should stop taking REVIA immediately and see your doctor as soon as possible.