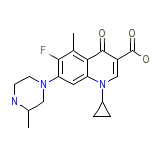
Raxar




Raxar Brand names, Raxar Analogs
Raxar Brand Names Mixture
- No information avaliable
Raxar Chemical_Formula
C19H22FN3O3
Raxar RX_link
http://www.rxlist.com/cgi/generic2/grepa.htm
Raxar fda sheet
Raxar msds (material safety sheet)
Raxar Synthesis Reference
No information avaliable
Raxar Molecular Weight
359.395 g/mol
Raxar Melting Point
No information avaliable
Raxar H2O Solubility
No information avaliable
Raxar State
Solid
Raxar LogP
2.119
Raxar Dosage Forms
Tablet
Raxar Indication
For treatment of adults with mild to moderate infections caused by susceptible strains of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis.
Raxar Pharmacology
No information avaliable
Raxar Absorption
Rapidly and extensively absorbed following oral administration. The absolute bioavailability is approximately 70%.
Raxar side effects and Toxicity
Withdrawn from the US market in 1999 due to associations with QTc prolongation and adverse cardiovascular events.
Raxar Patient Information
No information avaliable
Raxar Organisms Affected
Enteric bacteria and other eubacteria