Panurin




Panurin Brand names, Panurin Analogs
- Acuretic
- Aldactazide
- Aldoril
- Apresazide
- Aquarills
- Aquarius
- Bremil
- Caplaril
- Capozide
- Chlorosulthiadil
- Chlorothiazide
- Chlorsulfonamidodihydrobenzothiadiazine Dioxide
- Chlorzide
- Cidrex
- Dichlorosal
- Dichlorotride
- Dichlotiazid
- Dichlotride
- Diclotride
- Dicyclotride
- Dihydrochlorothiazid
- Dihydrochlorothiazide
- Dihydrochlorothiazidum
- Dihydrochlorurit
- Dihydrochlorurite
- Dihydroxychlorothiazidum
- Direma
- Disalunil
- Diu-Melusin
- Diuril
- Drenol
- Dyazide
- Esidrex
- Esidrix
- Esimil
- Fluvin
- HCTZ
- HCZ
- Hidril
- Hidrochlortiazid
- Hidroronol
- Hidrotiazida
- Hydril
- Hydro-Aquil
- Hydro-D
- Hydro-Diuril
- Hydrochlorothiazid
- Hydrochlorothiazide Intensol
- Hydrochlorthiazide
- Hydrodiuretic
- Hydrodiuril
- Hydropres
- Hydrosaluric
- Hydrothide
- Hydrozide
- Hypothiazid
- Hypothiazide
- Hyzaar
- Idrotiazide
- Inderide
- Ivaugan
- Jen-Diril
- Lopressor Hct
- Lotensin Hct
- Maschitt
- Maxzide
- Megadiuril
- Microzide
- Moduretic
- Nefrix
- Neo-Codema
- Neoflumen
- Newtolide
- Oretic
- Palonyl
- Panurin
- Perovex
- Primogyn
- Prinzide
- Ro-Hydrazide
- Servithiazid
- Thiaretic
- Thiuretic
- Thlaretic
- Timolide
- Unipres
- Urodiazin
- Vaseretic
- Vetidrex
- Ziac
- Zide
Panurin Brand Names Mixture
- No information avaliable
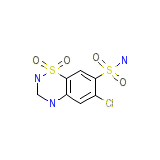
Panurin Chemical_Formula
C7H8ClN3O4S2
Panurin RX_link
http://www.rxlist.com/cgi/generic/hctz.htm
Panurin fda sheet
Panurin msds (material safety sheet)
Panurin Synthesis Reference
Werner et al., J. Am. Chem. Soc. 82, 1161 (1960)
Panurin Molecular Weight
297.741 g/mol
Panurin Melting Point
274 oC
Panurin H2O Solubility
0.7 mg/mL
Panurin State
Solid
Panurin LogP
-0.268
Panurin Dosage Forms
Tablet (oral)
Panurin Indication
For the treatment of high blood pressure and management of edema.
Panurin Pharmacology
Thiazides such as hydrochlorothiazide promote water loss from the body (diuretics). They inhibit Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.
Panurin Absorption
50-60%
Panurin side effects and Toxicity
The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in the mouse and rat.
Panurin Patient Information
General
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
Panurin Organisms Affected
Humans and other mammals